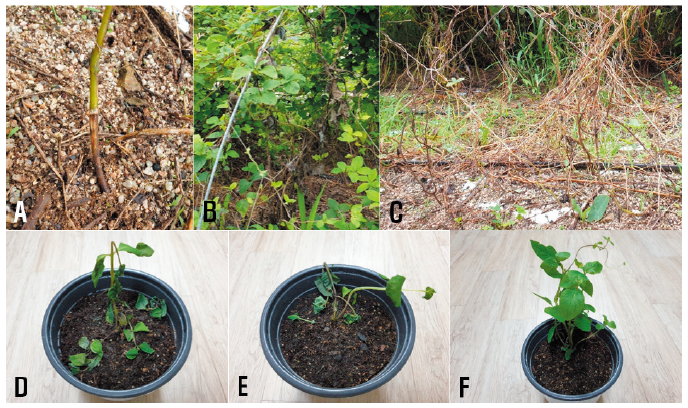
Bonnet bellflower (Codonopsis lanceolata (Siebold & Zucc.) Benth. &Hook.f. ex Trautv.) belongs to the family Campanulaceae and its native distribution ranges from East Russia to China, Korea, and Japan [1]. It is cultivated in Korea as a wild vegetable and medicinal plant. In July and September 2020, a severe outbreak of stem rot was observed in bonnet bellflower plants in a farm located in Chuncheon, Gangwon Province, Korea. The symptoms initially appeared on the stem at or above the soil line. The infected parts of the stem turned dark brown to black in color and rotted (Fig. 1A). With the progression of the disease, the infected stem completely rotted and blighted (Fig. 1B) and severely diseased plants died (Fig. 1C). Three sites in the field were designated to investigate the disease incidence and fifty plants from each site were investigated for the disease incidence. The incidence of diseased plants in the field was 2‒30%.
The diseased stems of bonnet bellflower plants were collected and the fungal pathogen was isolated from the stem lesions. The 3–5 mm-long lesion pieces cut from the diseased stems were surface-sterilized with 1% sodium hypochlorite solution for 1 min and plated on 2% water agar. The fungal mycelia growing from the lesion pieces were transferred to potato dextrose agar (PDA) slants after incubating the plates at 25℃ for one day. Ten isolates of Rhizoctonia sp. were obtained from the stem lesions (Table 1) and examined for their morphological characteristics using a compound microscope (Nikon Eclipse Ci-L, Japan). All isolates were identified as Rhizoctonia solani Kühn as per descriptions from previous studies [2, 3].
Table 1. List of Rhizoctonia sp. isolates from bonnet bellflower plants in a field in Chuncheon, Korea in July and September 2020.
|
The R. solani isolates were tested for the identification of anastomosis groups using tester isolates of R. solani (AG-1 through AG-5) as previously described [4, 5]. All tested isolates were classified as R. solani AG-4. Anastomosis reactions between the tested isolate and the tester isolate of R. solani AG-4 are shown in Fig. 2A. The colony of the isolates cultured on PDA displayed whitish light brown in color (Fig. 2B). Sclerotia were absent or rarely formed on the medium.
Three isolates of R. solani AG-4 were tested for pathogenicity on bonnet bellflower plants via artificial inoculation. Mycelial disks of 6 mm in diameter cut from the margins of actively growing cultures of each isolate on PDA were placed on the stems at the soil surface level of 56-day-old bonnet bellflower plants grown in circular plastic pots (height: 13.5 cm; upper diameter: 15 cm; lower diameter: 10 cm) in a vinyl greenhouse. Inoculated plant pots were placed in plastic boxes (71.0 cm × 53.5 cm × 40.5 cm) under 100% relative humidity at room temperature (24‒26℃) for 2 days. Thereafter, the inoculated plant pots were taken out of the plastic boxes and kept indoors at room temperature. The pathogenicity of isolates was rated based on the degree of stem rot symptoms 5 days after inoculation. The inoculation test was conducted in triplicate, with one plant per replicate.
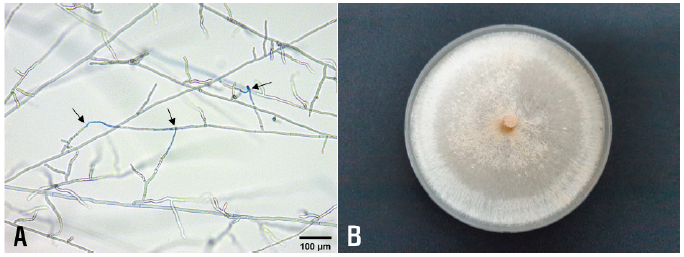
Fig. 2. Anastomosis test of Rhizoctonia solani isolate from bonnet bellflower and cultural appearance of the isolate. A: Anastomosis reactions between the tested isolate (left) and the tester isolate (right) of R. solani AG-4 observed by compound microscope. The arrows indicate points of hyphal anastomosis. B: A colony of R. solani AG-4 isolate grown on potato dextrose agar at 25℃ for 10 days.
All tested isolates of R. solani AG-4 induced stem rot symptoms in the inoculated plants (Figs. 1D and 1E), but no symptoms were observed in the control plants (Fig. 1F). The symptoms induced by the artificial inoculation of plants were similar to those observed in plants from the investigated field. The inoculated isolates were re-isolated from the lesions.
R. solani causes various diseases in many crops [6-8], and anastomosis groups of the fungus have different genetic and pathological characteristics [3]. Stem rot on bonnet bellflower caused by R. solani has previously been recorded in Korea [9]. However, there has been no report of disease incidence elsewhere. In addition, the anastomosis groups and pathogenicity of R. solani isolates from bonnet bellflower have not been previously reported. This is the first report of R. solani AG-4 causing stem rot in bonnet bellflower.