Introduction
The genus Aspergillus, a member of the Aspergillacea family, is the best-known fungi in the phylum Ascomycota, along with the genus Penicillium and Talaromyces [1]. As the largest phylum in the fungal kingdom, Ascomycota includes more than 64,000 species.
Since Pier Antonio Micheli introduced the first Aspergillus species in 1729 [2], over 900 species of Aspergillus have been reported [3]. In Korea alone, 69 Aspergillus species have been discovered, reflecting its diversity [4].
Aspergillus species are commonly used in agriculture, industry, human health, and plant physiology. Other Aspergillus species, such as A. niger, are pathogenic [2,5]. The name Aspergillus stems from the observation that its conidiophores form shapes that are reminiscent of the aspergillums used in Christian church rituals. Macroscopic and microscopic features are primarily used to characterize and analyze this fungal group [6]. Most of the members of this genus are exist in asexual states, forming asexual haploid conidia. Some species have been identified in a sexual state and form non-motile spores known as ascospores. Aspergillus species are aerobic and found in almost all oxygen-rich environments [2,7].
With the discovery of more species, it is no longer sufficient to rely on morphological features alone for identification [8], molecular analysis has been added to the regimen of conventional studies. Molecular identification of Aspergillus species depends on characterizing the internal transcribed spacer (ITS) region and DNA sequences of β-tubulin (BenA), calmodulin (CaM), and the second-largest subunit of RNA polymerase II (RPB2) [8,9]. This study used previously published sequences of BenA and CaM for molecular analysis [10,11].
In 2018, over 200 endophytic fungi were collected from plant roots in Gyeongsang Province, including Pohang, Jinju, Gumi, Gyeongsan, and Ulleungdo Island [12]. Among the isolates were two previously unreported Aspergillus species, named A. insuetus and A. nomius. This article describes the morphological and molecular features of these fungi found in Korea.
Materials and Methods
Isolation of endophytic fungi
During an extensive investigation of fungal diversity in Gyeongsang Province, Korea, in 2018, two previously unreported species of Aspergillus, named KMG411 and KMG412, were identified (Table 1 and Table 2) [12]. KMG411 was isolated from Lespedeza cuneate, and KMG412 from Chrysanthemum lavandulifolium. The collected roots were washed with distilled water to eliminate dust. After treatment with Tween-80 solution for 5 min, the root’s surface was sterilized with 1% perchloric acid. Washed roots were cut into pieces and incubated at 25℃ in Hagem minimal medium with 80 parts per million (ppm) of streptomycin [13,14]. Pure fungal strains were transferred and cultured on potato dextrose agar (PDA) from the root pieces [15]. The isolated strains were stored in 20% glycerol at -80℃ and deposited at the National Institute of Biological Resources (NIBR), Incheon, Korea, with the accession numbers NIBRFG0000505325 for KMG411 and NIBRFG0000505326 for KMG412.
Morphological analysis
Morphological characteristics of the two isolates were analyzed after growth on malt extract agar (MEA; Samson, 2010), Czapek yeast autolysate agar (CYA; Pitt, 1979), and yeast extract sucrose agar (YES; Frisvad, 1981) [10]. All plates were incubated at 25℃ in the dark for 7 days; the CYA plates underwent additional incubation at 30℃ and 37℃. Morphological characteristics, including the diameter and visible features of the fungal colonies, were observed. Fungal structures were measured and characterized by light microscopy (Eclipse 80i, Nikon, Tokyo, Japan) [16,17]. The specimens were fixed on glass slides with 85% and 99% lactic acid.
DNA extraction, PCR, and sequencing
The Accuprep® genomic DNA extraction kit (BIONEER Corp, Daejeon, USA) was used to extract DNA from the fungal isolates. DNA was stored at -20℃ before amplification. PCR amplification primers for Calmodulin (CaM) and β-tubulin (BenA), CMD5/CMD6 and Bt2a/Bt2b, were constructed based on prior publications by doctor Lee’s team and doctor Lim’s team [10,11]. After amplification in a 20-μL volume, a QIAquick PCR purification kit (Qiagen) was used to purify the PCR amplicons. Sequencing was performed on an ABI 310 DNA sequencer (Perkin Elmer, Foster, CA, USA) using the ABI PRISM BigDye Terminator Cycle Sequencing Kit (PE Biosystems, Foster, CA, USA) [1,18].
|
Table 1. Summary and GenBank accession numbers for Aspergillus strains isolated from Gyeongsang Province, Korea. 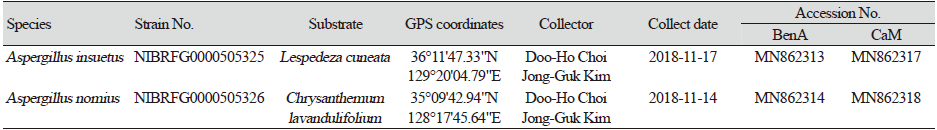
|
|
Table 2. Accession numbers for fungal strains used for the phylogenetic analysis. 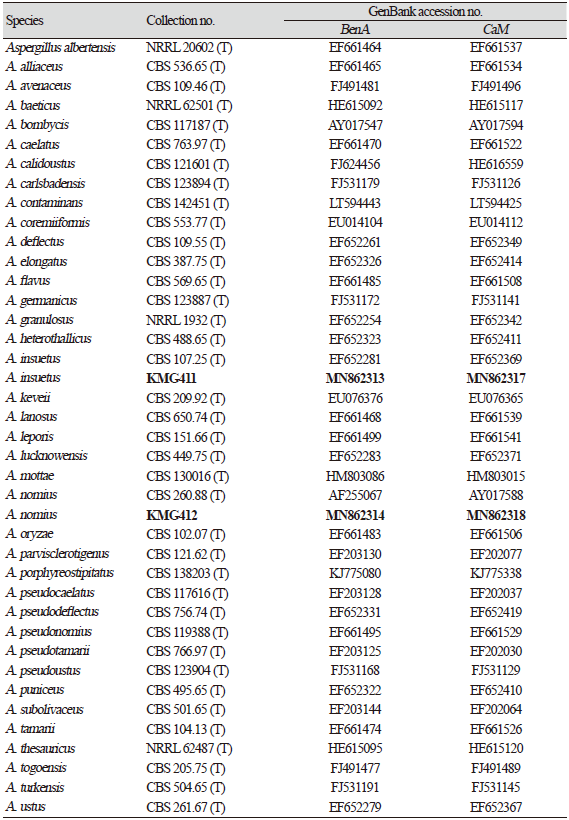
|
|
Bold letters indicate the strain and accession numbers of th eisolate identified in this study |
Phylogenetic analysis
Nucleotide sequences were aligned using BioEdit v7.2.5 (Clustal W) [19], then transferred to Molecular Evolutionary Genetics Analysis (MEGA) 7 software [20]. A phylogenetic tree was constructed based on the sequence similarities, a maximum likelihood (ML) using a Tamura 3-parameter model. The ML heuristic method was set to level 3 for subtree pruning regrafting (SPR), and bootstrap replicates were set to 1,000. The species similarity was calculated as a percentage based on a BLASTn search at the National Center for Biotechnology Information (NCBI) [16]. Two different groups were selected to construct the phylogenetic tree; A. versicolor for NIBRFG0000505325 [21] and A. muricatus for NIBRFG0000505326 [22].
Results and Discussion
Sequences of BenA (β-tubulin) and CaM (calmodulin) amplified from the isolated fungal strains were successfully identified through BLASTn-based comparisons and phylogenetic analyses. The gene sequences from the two Aspergillus species were identified in the GenBank database. Two isolates were included in monophyletic groups with the type strain for each Aspergillus species. The NIBRFG0000505325 isolate was homologous to the A. insuetus type strain (sequence similarities of 95.7-100% with EF652281 for BenA and 94.1-99.8% with EF652369 for CaM; bootstrap value of 99%). The NIBRFG0000505326 isolate was included in a monophyletic group with type strain A. nomius (sequence similarities of 98-99.6% with AF255067 for BenA and 98.9-99.4% with AY017588 for CaM; bootstrap value of 63%).
Currently, 72 species of Aspergillus have been revealed in Korea [4,10]. In this study, two Aspergillus species, named A. insuetus and A. nomius, were identified. To the best of our knowledge, these species have not been reported in Korea. Additional taxonomic information is presented in the following section.
Taxonomy
Aspergillus insuetus Thom & Church (1929), Mycobank No. 267997
Description: Colony diam, 7 d, in mm: CYA 28-31; CYA 30℃ 43-47; CYA 37℃ 5-9; MEA 20-24; YES 30-33 (Fig. 1 and Fig. 2).
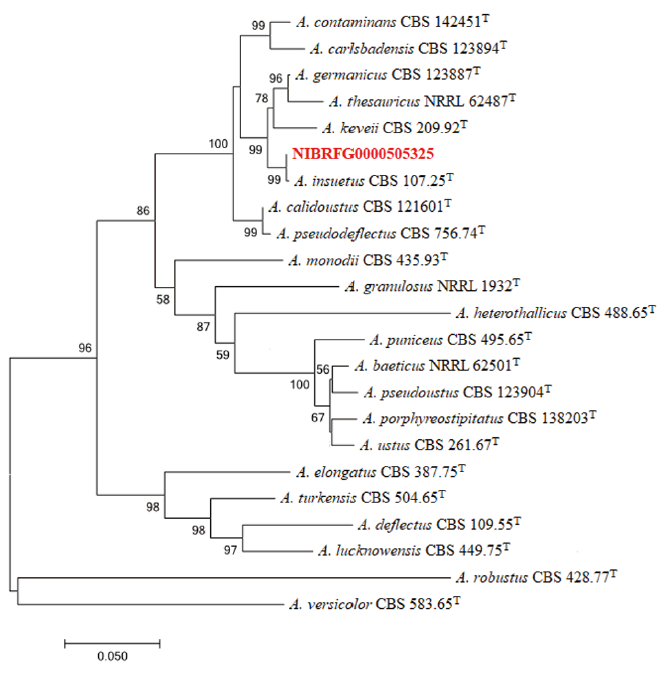
Fig. 1. Maximum-likelihood phylogenetic analysis of combined sequence data of BenA and CaM genes from NIBRFG0000505325. The sequence of A. versicolor was used as an outgroup. Bootstrap scores >50 are presented. The number of nucleotide substitutions per site was denoted by the scale bar. “T” identifies the type strains of the given fungal speciesT. he isolate NIBRFG0000505325 is marked in red.
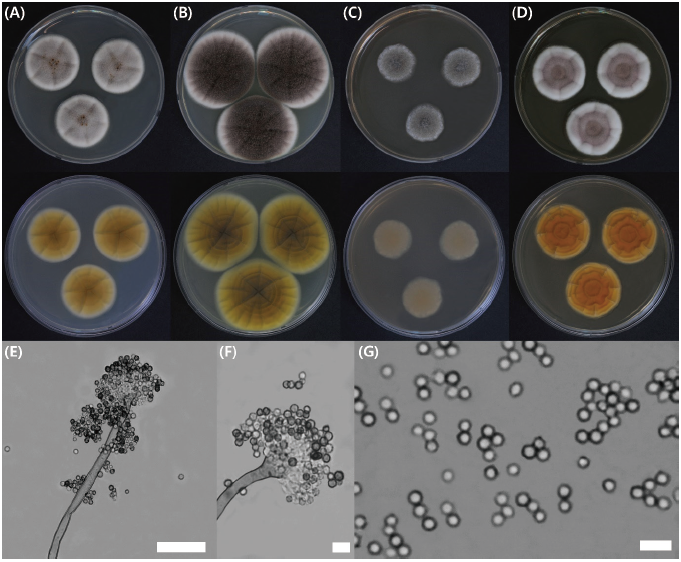
Fig. 2. Aspergillus insuetus KMG411 cultures after 7 days in culture. Colonies were grown on (A) Czapek yeast autolysate (CYA) agar at 25℃ and (B) 30℃, (C) on malt extract agar (MEA) at 25℃ and (D) yeast extract sucrose (YES) agar at 25℃; images include the top and undersides of the plates. (E-F) Conidiophores; (G) Conidia (scale bars; E, 50 μm; F and G, 10 mμ).
CYA, 25℃: Colonies moderately deep, angular shape, radially sulcate with a yellowish-gray color; margins low, narrow, entire; mycelia white; texture floccose; sporulation sparse, soluble pigment present, reverse pigmentation reddish-brown at center fading to pale.
MEA, 25℃: Colonies moderately deep, lightly sulcate with a dull greyish-green color; margins low, narrow, entire; mycelia white; texture floccose; sporulation dense at the center, soluble pigment absent, reverse pigmentation brown at center fading to pale.
YES, 25℃: Colonies moderately deep, lightly radially sulcate with a dull reddish-brown color; margins high, moderate, entire; mycelia white; texture floccose and velutinous; sporulation dense at margins, soluble pigment absent, reverse pigmentation deep reddish-orange at center fading to reddish-yellow.
Micromorphology: Conidiophores had a floccose conidial head, distinct rough-walled stipes, 6-8.4×280 μm; vesicle, roughened, globose to subglobose, 9-15 μm; phialides were ampulliform type, navicular shape, smooth; conidium globose to subglobose, color with echinulations, 3-3.8 μm; distinct roughened and inner and outer-walled.
Strain examined: NIBRFG0000505325, isolated from Lespedeza cuneatea in Pohang (36°11'47.33"N, 129°20'04.79"E).
Note: When compared with the type strain of Aspergillus insuetus, A. insuetus from Korea can be easily distinguished by colony growth on CYA at 37℃. Colony growth on other mediums showed similar growth in both species. Like type strain of A. insuetus, the isolated strain had a similar shape of conidial head, vesicle, and conidium, but was slightly smaller. Type A. keveii, close to type A. insuetus in the phylogenetic tree, had lower growth activity in medium and rough-walled stipes not smooth-walled [22,23].
Aspergillus nomius Kurtzman, B.W. Horn & Hesselt (1987), Mycobank No. 133392
Description: Colony diam, 7 d, in mm: CYA 52-55; CYA 30℃ 61-67; CYA 37℃ 53-57; MEA 56-62; YES 66-69 (Fig. 3 and Fig. 4).
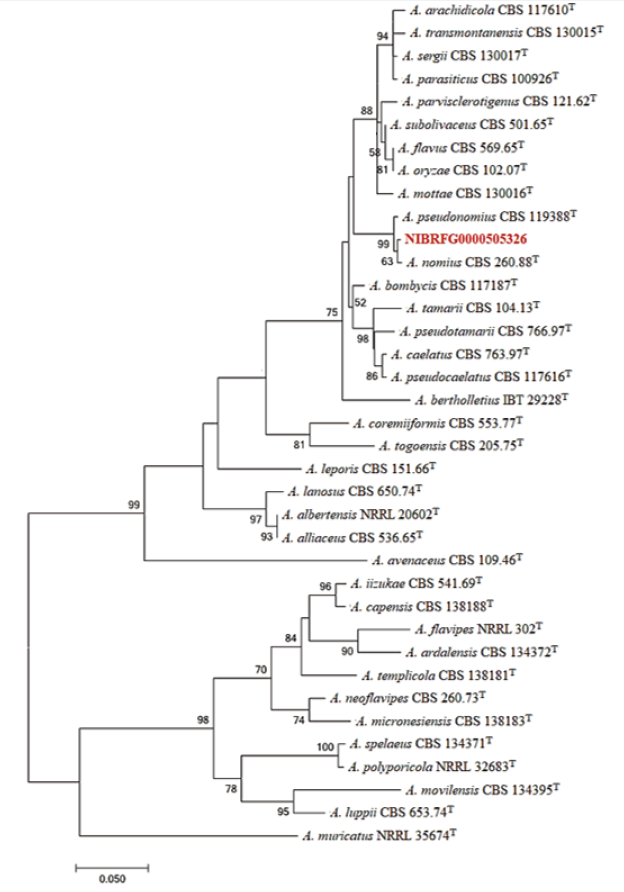
Fig. 3. Maximum-likelihood phylogenetic analysis of combined sequence data of BenA and CaM genes from NIBRFG0000505326. The sequence of A. muricatus was used as an outgroup. Bootstrap scores >50 are presented. The number of nucleotide substitutions per site was denoted by the scale bar. “T” identifies the type strains of the given fungal speciesT. he isolate NIBRFG0000505326 is marked in red.
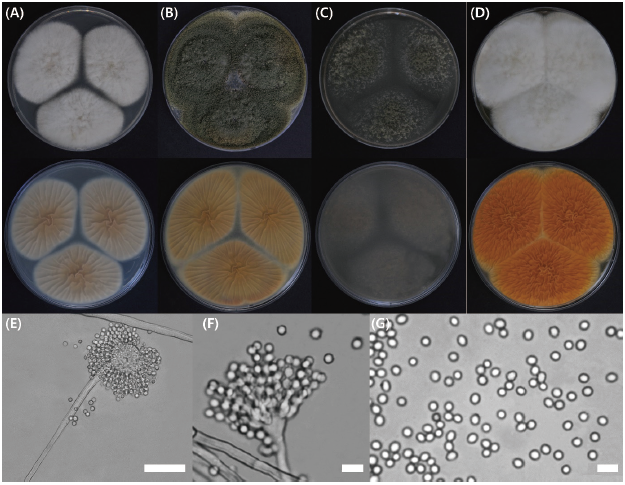
Fig. 4. Aspergillus nomius KMG412 after 7 days in culture. Colonies were grown on (A) Czapek yeast autolysate (CYA) agar at 25℃ and (B) 30℃, (C) on malt extract agar (MEA) at 25℃ and (D) yeast extract sucrose (YES) agar at 25℃; images include the top and undersides of the plates. (E-F) Conidiophores; (G) Conidia (scale bars; E, 50 μm; F and G, 10 mμ).
CYA, 25℃: Colonies highly deep, lightly radially sulcate, with a yellowish-white color; margins low, narrow, entire; mycelia white; texture floccose and velutinous; plentiful sporulation distributed evenly, soluble pigment absent, reverse pigmentation yellowish-brown at center fading to cream to brown.
MEA, 25℃: Colonies moderately deep, lightly sulcate with a dull greyish-green color; margins low, moderate, entire; mycelia pale greyish-yellow; texture floccose and velutinous; sporulation dense at the center, soluble pigment absent, reverse pigmentation pale greyish-green at center fading to translucent.
YES, 25℃: Colonies high and deep, randomly sulcate, have a dull reddish-white color; margins low, narrow, entire; mycelia yellowish-white; texture floccose and velutinous; sporulation dense at margins, soluble pigment absent, reverse pigmentation deep reddish-orange at center fading to reddish-yellow.
Micromorphology: Conidiophores with typically radiate conidial head, hyaline, distinct rough-walled stipes, over 1,000 μm; vesicle, roughened, globose to subglobose, 20-33 μm; phialides were ampulliform type, navicular shape, smooth; conidium globose to subglobose, color with pale green, 3-3.4 μm; distinct roughened and inner and outer-walled.
Strain examined: NIBRFG0000505326, isolated from Chrysanthemum lavandulifolium in Jinju (35°09'42.94"N, 128°17'45.64"E).
Note: When compared with the type strain of Aspergillus nominus, A. nominus from Korea can be easily distinguished because the isolated strain did not show small bullet-shaped sclerotia. Otherwise, both strains had similar growth activity on all media. Comparing with A. pseudonominus, A. nominus showed similar growth activity except on CYA at 25℃ and 37℃. In both conditions, A. nominus had slower growth than type strain A. pseudonominus. A. nominus also had larger conidiophores than type A. pseudonominus [24,25].
Acknowledgments
This study was supported by grants from the National Institute of Biological Resources (NIBR) and Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Environment (MOE) of the Republic of Korea NIBR201801208, the Ministry of Education (NRF-2016R1A6A1A05011910) and Research Institute for Dok-do and Ulleung-do Island of Kyungpook National University, Korea.


