Wasabi [Eutrema japonicum (Miq.) Koidz.], also called Japanese horseradish, belongs to the family Brassicaceae, and its native range is Japan, Korea, and Sakhalin [1]. The plant grows naturally along stream beds in mountains and is cultivated as a vegetable in East Asian countries. The plant has a hot and tangy flavor [2], and its leaves can be eaten as a green salad. The plant has been cultivated in vinyl greenhouses to harvest leaves in alpine areas of Korea.
In June 2021, foot rot symptoms were observed in wasabi plants growing in vinyl greenhouses of the Alpine Agricultural Experiment Station, Wild Vegetable Research Institute, in Taebaek, Gangwon Province, Korea. Diseased plants displayed black soft rot of crowns and petioles at the soil line and wilted (Figs. 1A‒C). Fifty plants in a vinyl greenhouse were investigated for the disease incidence. The incidence of diseased plants was 2‒10% in four out of five vinyl greenhouses investigated.
Fungal pathogens were isolated from diseased wasabi. The 3‒5 mm long lesion pieces cut from crown lesions of the diseased plants were plated on 2% water agar after surface sterilization with 1% sodium hypochlorite solution for 1 min. Single hyphal tips growing from the lesion pieces were transferred to potato dextrose agar (PDA) slants after incubating the plates at 25℃ for 2‒3 days. Eight isolates of Phytophthora sp. were obtained from the lesion pieces. Each isolate was cultured on PDA and V8-juice agar (V8A) in 9 cm diameter Petri dishes at 25°C in the dark for 10 days to investigate the cultural features. The colony morphology of the isolates showed a chrysanthemum-shaped growth pattern on PDA and a rose-shaped growth pattern on V8A (Figs. 2A and B).
Eight mycelial disks (10 mm in diameter) were cut and removed from the 10-day-old V8A cultures. To induce sporangia, ten milliliters of sterile distilled water was poured into the culture dishes, which were incubated at 25°C under fluorescent light for 7 days. The morphological characteristics of 20‒30 sporangia per isolate produced inside the eight mycelial disk holes in the culture dishes were examined using a compound light microscope (Nikon Eclipse Ci-L, Japan). The sporangia were non-papillate, ovoid, ellipsoid to pyriform with rounded bases, terminal, persistent on the sporangiophores (Figs. 2C and D), and measured 34.7‒54.2×27.0‒34.8 ㎛ (av. 44.4×29.5 ㎛). The morphological characteristics of the isolates were similar to those of Phytophthora pseudocryptogea Safaief., Mostowf., G.E. Hardy, and T.I. Burgess, described in a previous study [3].
Molecular identification was conducted to confirm the identification of P. pseudocryptogea isolates based on their morphological characteristics. Total genomic DNA was extracted using a HiGene Genomic DNA Prep Kit (BIOFACT, Daejeon, Korea), according to the manufacturer's protocol. DNA sequencing of partial of heat shock protein 90 (HSP90), beta tubulin (TUB), and cytochrome c oxidase subunit 1 (COX) genes was performed as previously described [4, 5]. As a result, 1,633, 812, and 864 bp were obtained for the HSP90, TUB, and COX genes, respectively, from the isolates. A neighbor-joining tree for the combined dataset was generated using MEGA X software [6]. All obtained sequences were identical to each other, and showed 99–100% similarity with P. pseudocryptogea SUC620. Phylogenetic analysis showed that all isolates were clustered in a group of P. pseudocryptogea strains (Fig. 3). The nucleotide sequences of the COX, HSP90, and TUB genes obtained from the eight isolates were registered in NCBI GenBank, numbered as LC699356–LC699363, LC699364–LC699371, and LC699372–LC699379, respectively.
Three isolates of P. pseudocryptogea were tested for pathogenicity in wasabi plants using artificial inoculation. Each isolate was cultured on V8A medium to prepare the inoculum. Twenty mycelial disks (10 mm in diameter) cut from 10-day-old V8A cultures were transferred into 9 cm diameter Petri dishes with 20 mL of sterile distilled water and incubated at 24–26°C under fluorescent light for 7 days to produce sporangia. The Petri dishes were placed in a refrigerator at 4°C for 2hr to induce the release of zoospores from the sporangia. Thereafter, the culture solution in the Petri dishes was filtered through four layers of gauze to prepare a zoospore suspension. The concentration of each zoospore suspension was adjusted to 105‒106 zoospores/mL. The zoospore suspension of each isolate was inoculated into 10-month-old wasabi plants grown in circular plastic pots (height: 15 cm; upper diameter: 17 cm; lower diameter: 10 cm) in a vinyl greenhouse. For the inoculation test, the surface soil around the plants in the pots was dug to a depth of 1–2 cm. Each zoospore suspension (60 mL) was poured around the plant in a pot and the inoculated plant part was covered with the original soil. The same quantity of sterile distilled water was used for the control plant. Inoculated plants were cultivated in a greenhouse at 24‒30℃. The inoculation test was performed in triplicates. The pathogenicity of the isolates was rated based on the degree of foot rot symptoms induced 10 days after inoculation. All the tested isolates of P. pseudocryptogea induced foot rot symptoms in the inoculated plants (Figs. 1D and E), but no symptoms were observed in the control plants (Fig. 1F). The symptoms induced by the artificial inoculation of plants were similar to those observed in plants from the investigated vinyl greenhouses. The inoculated isolates were re-isolated from the lesions.
Phytophthora cryptogea Pethybr. & Laff. has been reported to cause root and crown rot in wasabi in the USA [7], and Phytophthora drechsleri Tucker causes phytophthora rot in wasabi in Japan [8]. P. pseudocryptogea was first named based on the morphology and phylogenetic analysis of isolates belonging to the P. cryptogea species complex [3]. The pathogen has been reported to cause root rot in several plants, including Solanum melongena L. [9]. However, there are no reports of disease occurrence in wasabi caused by the pathogen. This is the first report of P. pseudocryptogea causing Phytophthora foot rot in wasabi.

Fig. 1.Foot rot symptoms in wasabi plants. A‒C: Symptoms observed in the vinyl greenhouse investigated. D and E: Symptoms induced by artificial inoculation tests with Phytophthora pseudocryptogea isolates. F: A non-inoculated plant.
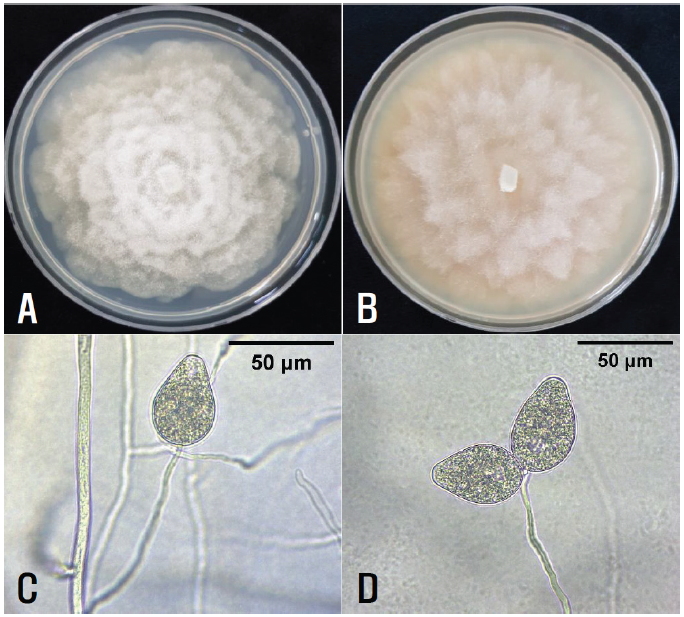
Fig. 2.Cultural and morphological features of Phytophthora sp. isolates from diseased wasabi plants. A and B: Colonies of the isolate grown on potato dextrose agar (A) and V8-juice agar (B) at 25°C for 10 days. C and D: Hyphae, sporangiophores, and sporangia of the isolates.

Fig. 3.Neighbor-joining phylogenetic tree based on a concatenated alignment of partial of heat shock protein 90, beta tubulin, and cytochrome c oxidase subunit 1 sequences of five Phytophthora cryptogea isolates (SDPHY-2001‒SDPHY-2005) from wasabi and reference species. The reference sequence data were obtained from the NCBI GenBank database. The bootstrap support values are given at the nodes. The bar represents the number of nucleotide substitutions per site.
Conflict of Interests
No conflict of interest was reported by the author(s).
Acknowledgement
This study was supported by a research grant (PJ014507012020) from the Rural Development Administration, Korea.
