Korean Journal of Mycology (Kor J Mycol) 2023 September, Volume 51, Issue 3, pages 177. https://doi.org/10.4489/KJM.20230019
Received on June 23, 2023, Revised on September 18, 2023, Accepted on October 05, 2023.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
INTRODUCTION
Macrofungi play important roles in natural ecosystems, adopting saprophytic, parasitic, and symbiotic lifestyle. They encompass ascomycetes and basidiomycetes, which are characterized by conspicuous sporebearing structures that are easily observable [1]. While the worldwide compilation of macrofungi names by the Index fungorum exceeds 20,000, the recorded macrofungi species in Korea amount to approximately 2,100. Among these, 1,800 species were basidiomycetes fungi and 290 species were ascomycetes fungi [2].
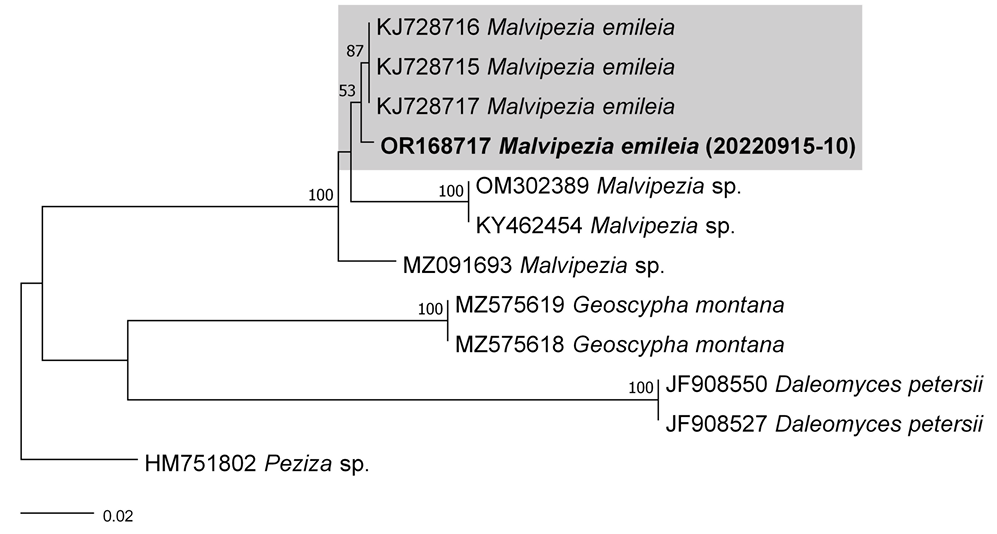
In this study, we conducted taxonomic research on the diversity of wild macrofungi in the forests of South Korea. Four previously unrecorded macrofungal species were collected in 2022. Based on taxonomic identification (morphological features and DNA fungal barcodes), two basidiomycete fungi (Laccaria striatula and Xerula strigosa) and two ascomycete fungi (Leotia atrovirens and Malvipezia emileia) were confirmed. Among these, Malvipezia was discovered to be an unknown genus in South Korea.
Section
Four samples were collected during a mycological survey conducted in 2022 to investigate the mushroom diversity. In this study, these collections were examined for morphological identification based on their macroscopic and microscopic characteristics. The dried materials were mounted in distilled water and 5% KOH using a Zeiss Axio Imager A1 microscope (Jena, Germany) and an Axiocam 503 color camera (Jena, Germany). The taxonomic classification of the studied taxa followed the guidelines provided by the Index Fungorum (http://www.indexfungorum.org). Dried specimens were archived at the herbarium of the National Institute of Biological Resources (NIBR), Incheon, South Korea.
Table 1 Specimens of species used in this study and their GenBank accession numbers.
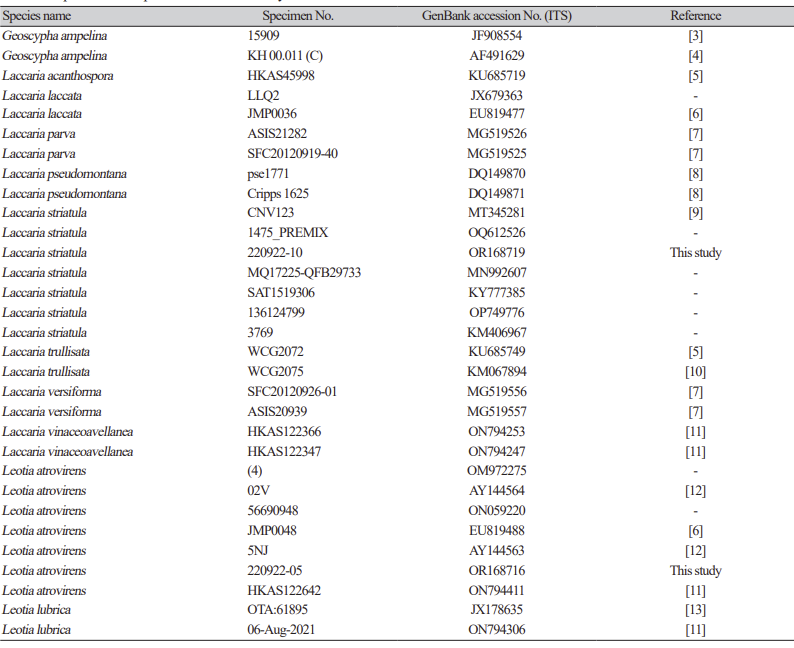
Table 1 Specimens of species used in this study and their GenBank accession numbers (continued).
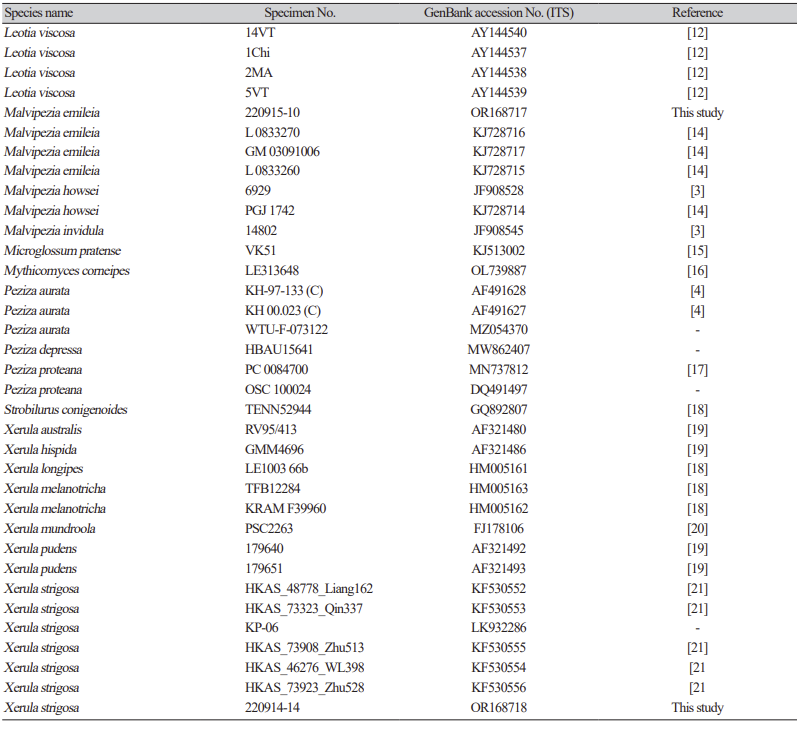
Section
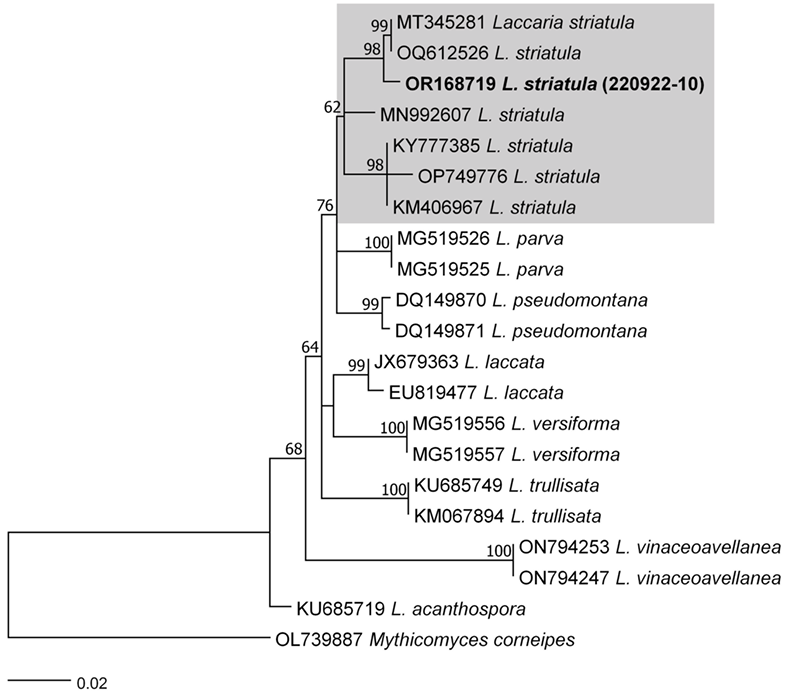
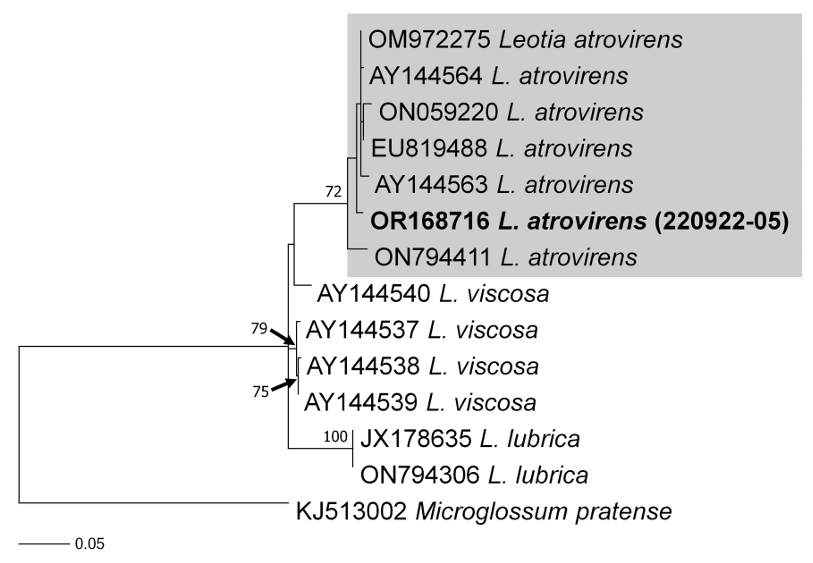
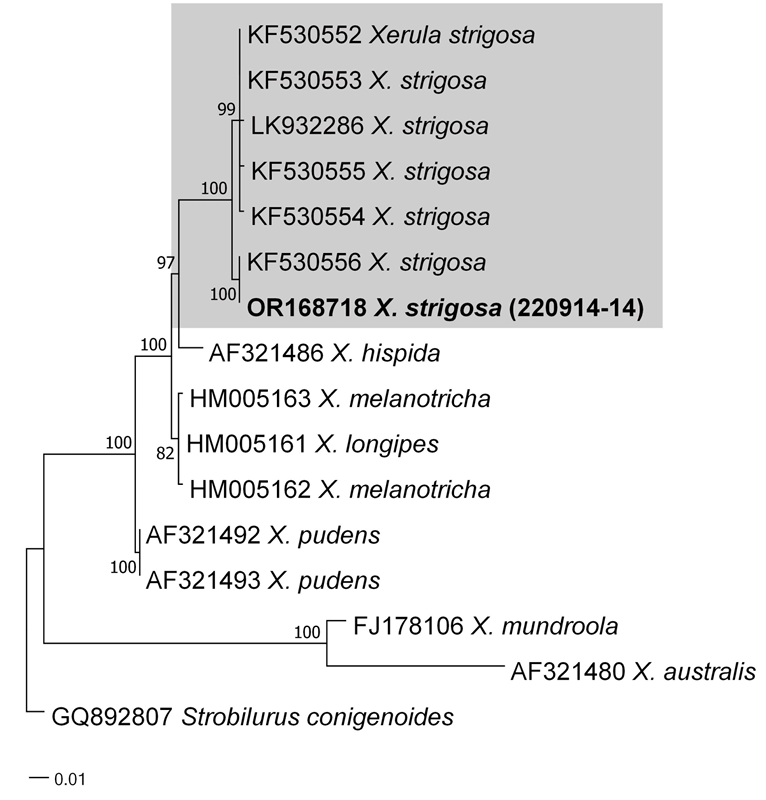
As a result, the ITS sequences were subjected to an RaxML analysis, resulting in the resolution of the phylogenetic positions of four species (Laccaria striatula, Leotia atrovirens, Malvipezia emileia, Xerula strigosa) (Figs. 1-4). Each species formed well-supported clades in their respective phylogenetic trees. In Laccaria phylogeny (Fig. 1), the Korean collection grouped together with L. striatula. Within the L. striatula clade, this species exhibited three distinct groups, indicating the presence of a species complex, based on ITS sequences. Further studies are required to validate the phylogenetic position of L. striatula. Regarding the Leotia phylogeny (Fig. 2), the Korean specimen was clustered with L. atrovirens group. Based on the phylogenetic analysis, the Korean collection was conclusively identified as L. atrovirens. In Malvipezia phylogeny (Fig. 3), the Korean collection clustered with M. emileia (KJ728715-KJ728717) with 100% BS values. Thus, the phylogenetic tree we constructed was supported by the position of M. emileia. In Xerula phylogeny (Fig. 4), X. strigosa clade was split into two groups. The Korean specimens clustered with X. strigosa from China (KF530556), with 100% BS values. Further studies are required to confirm this differentiation within X. strigosa clade.
TAXONOMY
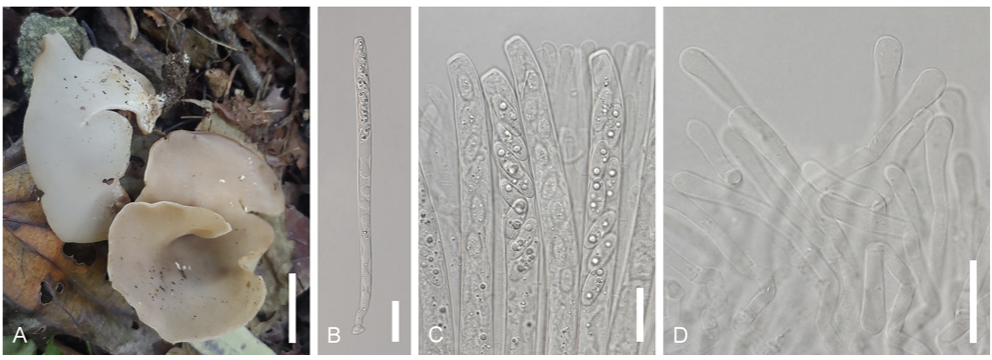
Laccaria striatula (Peck) Peck. Bull. N.Y. St. Mus. 157: 93 (1912) [1911] (Figs. 1 and 5)
Description: Pileus: Diameter ranging from 15 to 40 mm., convex to flattened, with flesh pink to pinkish brown coloration, striated. Lamellae: Adnate, thick, orange-pinkish. Stipe: Colored in shades of orange, pinkish, or brown, finely fibrillose, 20–50 mm × 2–4 mm. Basidia: Clavate-shaped, 4-spored, with dimensions of 53–68 × 12–14.5 μm. Basidiospores: Globose shape, echinulate with spines, measuring 8.012.0 × 9.0–11.5 μm; Q = 0.9–1.3; n = 20. Cheilocystidia rarely observed, filamentous, flexuous. Pileipellis a cutis, hyphae interwoven, cylindrical, bifurcating, clamped. Pileocystidia not observed.
Habitat: Found on the ground of the forests.
Specimen examined: Location: Gangwon-do, Wonju-si, Korea. Coordinates: 37°21ʹ47.75ʺN
Specimen examined: Location: Gangwon-do, Wonju-si, Korea. Coordinates: 37°25ʹ12.12ʺN 128°3ʹ13.53ʺE, alt. 322 m. Collection date: Sep. 14, 2022. Specimen voucher: CKU20220914-14.
Remarks. The description based on the Korean collection is in accordance with the holotype description [28]. Xerula strigosa has been documented in both China and Pakistan as well. Hence, considering its morphological features and molecular analysis, this represents the species’ third recorded occurrence.
REFERENCE
Mueller GM, Schmit JP, Leacock PR. et al. Global diversity and distribution of macrofungi. Biodivers Conserv 2007;16:37–48.
https://doi.org/10.1007/s10531-006-9108-8
NIBR. 2022. National Institute of Biological Resources. Available at: https://kbr.go.kr/ [Date accessed: 23 March 2022]
Garbelotto MM, Baker LJ, Smith A, Robich G, Osmundson T, Mizzan L. Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PLoS ONE 2013;8(4):E62419.
https://doi.org/10.1371/journal.pone.0062419
Hansen K, Læssøe T, Pfister DH. Phylogenetic diversity in the core group of Peziza inferred from ITS sequences and morphology. Mycol Res 2002;106(8):879-902.
https://doi.org/10.1017/S0953756202006287
Wilson AW, Hosaka K, Mueller GM. Evolution of ectomycorrhizas as a driver of diversification and biogeographic patterns in the model mycorrhizal mushroom genus Laccaria. New Phytol 2017;213(4): 1862-1873.
https://doi.org/10.1111/nph.14270
Palmer JM, Lindner DL, Volk TJ. Ectomycorrhizal characterization of an American chestnut (Castanea dentata)-dominated community in Western Wisconsin. Mycorrhiza 2008;19(1):2736.
https://doi.org/10.1007/s00572-008-0200-7
HJ Cho, MS Park, H L, S-Y Oh, AW Wilson, GM Mueller, YW Lim. A systematic revision of the ectomycorrhizal genus Laccaria from Korea. Mycologia 2018;110(5):948-961.
https://doi.org/10.1080/00275514.2018.1507542
Osmundson TW, Cripps CL, Mueller GM. Morphological and molecular systematics of Rocky Mountain alpine Laccaria. Mycologia 2005;97(5):949-972.
https://doi.org/10.1080/15572536.2006.11832746
Victoroff CN. Response of ectomycorrhizal fungal fruiting to nitrogen and phosphorus additions in bartlett experimental forest, new Hampshire. Department of Environmental and Forest Biology. Master of Science Degree. State University of New York. 2022. 103 pp.
Wilson AW, Wickett NJ, Grabowski P, Fant J, Borevitz J, Mueller GM. Examining the efficacy of a genotyping-by-sequencing technique for population genetic analysis of the mushroom Laccaria bicolor and evaluating whether a reference genome is necessary to assess homology. Mycologia 2015;107(1):217-226.
https://doi.org/10.3852/13-278
Wang R, Herrera M, Xu W, Zhang P, Moreno JP, Colinas C, Yu F. Ethnomycological study on wild mushrooms in Pu'er Prefecture, Southwest Yunnan, China. J Ethnobiol Ethnomed 2022;18(1):55.
https://doi.org/10.1186/s13002-022-00551-7
Zhong Z, Pfister DH. Phylogenetic relationships among species of Leotia (Leotiales) based on ITS and RPB2 sequences. Mycol Prog 2004;3(3):237-246.
https://doi.org/10.1007/s11557-006-0094-8
Teasdale SE, Beulke AK, Guy PL, Orlovich DA. Environmental barcoding of the ectomycorrhizal fungus Cortinarius. Fungal Divers 2013;58(1):299-310.
https://doi.org/10.1007/s13225-012-0218-1
Medardi G, LoBuglio KF, Pfister DH, Lantieri A. Morphological and molecular study of Peziza emileia and P. howsei, two distinct taxa. Mycol Prog 2014;13:1227-1234.
https://doi.org/10.1007/s11557-014-1013-z
Kucera V, Lizon P, Tomsovsky M. Taxonomic divergence of the green naked-stipe members of the genus Microglossum (Helotiales). Mycologia 2017;109(1):46-54.
https://doi.org/10.1080/00275514.2016.1274620
Malysheva EF, Kiyashko AA, Malysheva VF, Shikalova EA. A survey of rare species of agaricoid fungi (Basidiomycota) from South Siberia, Russia. Turczaninowia 2022;25(1):52-72.
https://doi.org/10.14258/turczaninowia.25.1.6
Vizzini A, Medardi G, Tamm H, Forin N, Ercole E. Study and clarification of Peziza petersii and P. proteana (Ascomycota, Pezizales, Pezizaceae), and the status of the ‘cabbage-head fungus’ (P. proteana f. sparassoides). Mycol Prog 2020;19(5):505-523.
https://doi.org/10.1007/s11557-020-01575-7
Petersen RH, Hughes KW. The Xerula/Oudemansiella Complex (Agaricales). Nova Hedwig Beih 2010;137:625.
Mueller GM, Wu Q-X, Huang Y-Q, Guo S-Y, Aldana-Gomez R, Vilgalys R. Assessing biogeographic relationships between North American and Chinese macrofungi. J Biogeogr 2001;28(2):271-281.
https://doi.org/10.1046/j.1365-2699.2001.00540.x
Lebel T, Catcheside PS. The truffle genus Cribbea (Physalacriaceae, Agaricales) in Australia. Aust Syst Bot 2009;22:39-55.
https://doi.org/10.1071/SB07041
Qin J, Hao Y-J, Li YC, Feng B, Hao YJ. Paraxerula ellipsospora, a new Asian species of Physalacriaceae. Mycol Prog 2014;13(3):639-647.
https://doi.org/10.1007/s11557-013-0946-y
Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014;30(9):1312-1313.
https://doi.org/10.1093/bioinformatics/btu033
Miller MA, Pfeiffer W, Schwartz T. "Creating the CIPRES Science Gateway for inference of large phylogenetic trees" in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA. pp 1–8.
https://doi.org/10.1109/GCE.2010.5676129
Mueller GM. Laccaria laccata complex in North America and Sweden: Intercollection pairing and morphometric analyses. Mycologia 1991;83(5):578-594.
https://doi.org/10.1080/00275514.1991.12026057
HJ Cho, H L, MS Park, KH Park, JH Park, YH Cho, CM Kim, YW Lim. Two new species of Laccaria (Agaricales, Basidiomycota) from Korea. Mycobiology 2020;48(4):288-295.
https://doi.org/10.1080/12298093.2020.1786961
Geoffrey K. An Illustrated Guide to Mushrooms and Other Fungi of North America. Stamford, Connecticut: Lubrecht & Cramer Ltd. 1994. p.178.
Pfister DH, Healy R, Furci G, Mujic A, Nouhra E, Truong C, Smith MEA. A reexamination and realignment of Peziza sensu lato (Pezizomycetes) species in southern South America. Darwiniana nueva serie 2022;10(1):236-281.
https://doi.org/10.14522/darwiniana.2022.101.1019
Wang Lan, Yang Zhu-Liang, Zhang Li-Fang, Mueller GM. Synopsis and systematic reconsideration of Xerula s. str. (Agaricales). Plant Divers 2008;30(6):631-644.