Gyo-Bin Lee, Weon-Dae Cho, and Wan-Gyu Kim*
Global Agro-Consulting Corporation, Suwon 16614, Korea
*Correspondence to wgkim5121@naver.com
Korean Journal of Mycology (Kor J Mycol) 2024 September, Volume 52, Issue 3, pages 189-193.
https://doi.org/10.4489/kjm.520304
Received on May 24, 2024, Revised on September 21, 2024, Accepted on September 21, 2024, Published on Sep 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
During a disease survey conducted in March 2024, gray mold symptoms were observed in stringy stonecrop (Sedum sarmentosum) plants grown in vinyl greenhouses in Icheon, Korea. The symptoms included water-soaked lesions and soft rot on the stems and leaves. A large amount of gray mold formed on the lesions during the later stages of disease progression. The gray mold affected 5–20% of the plants in 8 out of 20 surveyed vinyl greenhouses. Three single-conidium fungal isolates were obtained from the lesions and identified as Botrytis cinerea through morphological characterization and phylogenetic analysis. The pathogenicity of the B. cinerea isolates was confirmed by artificial inoculation into stringy stonecrop plants, producing symptoms similar to those observed in the vinyl greenhouses. This is the first report of B. cinerea causing gray mold in stringy stonecrop.
Botrytis cinerea, Gray mold, Sedum sarmentosum, Stringy stonecrop
Stringy stonecrop (Sedum sarmentosum Bunge), a creeping perennial succulent plant in the family Crassulaceae, is native to East Asia [1] and thrives in various temperate conditions. This plant has been investigated for medicinal purposes in China [2] and is used as a culinary vegetable in Korea.
During a disease survey conducted in March 2024, weakened, discolored, and blighted stringy stonecrop plants were found in vinyl greenhouses in Icheon, Korea (Fig. 1A). Water-soaked lesions and soft rot were observed on the stems and leaves near the soil line, with gray mold forming on the lesions during the later stages of disease progression (Fig. 1B). The incidence of gray mold was assessed in 100 plants at each of three sites within a vinyl greenhouse. Results revealed that gray mold affected 5‒20% of the plants in 8 out of 20 surveyed vinyl greenhouses.
Samples of diseased plants were collected to isolate fungal pathogens. Conidia were harvested from lesions, and a conidial suspension was prepared with sterile distilled water. The conidial suspension was streaked onto 2% water agar (WA; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) using a sterile loop, and the WA plates were incubated at 20℃ for 1 day. A germinated conidium was transferred to WA and incubated at 20℃ for 5 days to obtain single-conidium isolate. Three isolates (SSBC-2401, SSBC-2404, and SSBC-2405) were selected for morphological characterization, phylogenetic analysis, and pathogenicity testing.

Fig. 1. Gray mold symptoms on stringy stonecrop plants. A and B: Symptoms observed on stems and leaves in the vinyl greenhouse. C: Symptoms induced on stems and leaves following artificial inoculation with Botrytis cinerea isolate SSBC-2405. D: Non-inoculated control plants.
The isolates were cultured on potato dextrose agar (PDA; BD Difco, Sparks, USA) in 9-cm Petri dishes at 20℃ in the dark for 21 days. Colonies of the isolates displayed white to gray mycelia and black, spherical or irregular sclerotia (1‒6 mm in diameter) (Fig. 2A). The morphology of 30 conidia and 25 conidiophores of each isolate was examined using a light microscope (Nikon Eclipse Ci-L, Tokyo, Japan). Conidiophores were erect, more than 1 mm in length, 10‒20 μm thick, branched, and pale brown (Fig. 2B). Conidia were hyaline to pale brown, globose to ovoid, and measured 6.8‒13.8 × 5.4‒9.6 μm (mean 9.2 × 6.8 μm) (Fig. 2B, 2C). The isolates were morphologically similar to Botrytis cinerea Pers. described by Ellis [3].
To verify the morphological identification, phylogenetic analysis was conducted. Genomic DNA of the isolates was extracted following a previously described method [4], with slight modifications. Polymerase chain reaction (PCR) amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), heat-shock protein 60 (HSP60), and RNA polymerase II second largest subunit (RPB2) gene regions was performed using primer sets from a previous study [5]. The PCR products were generated using DNA Free-Multiplex Master Mix (Cellsafe, Korea) and purified with ExoSAP-IT™ (Applied Biosystems, USA). Sequencing was conducted by Bionics Co., Ltd. (Seoul, Korea), and sequences were manually edited in SeqMan II (DNASTAR Inc., Madison, WI, USA) if necessary. Sequences of the isolates were aligned with other Botrytis spp. [5,6] using MUSCLE [7] and refined in MEGA version 7 [8]. Neighbor-joining (NJ) analysis of concatenated alignments was performed using the maximum composite likelihood model with 1,000 bootstrap replicates in MEGA version 7 [8]. Sclerotinia sclerotiorum 484 [5] served as the outgroup. Phylogenetic analysis showed that the isolates clustered with two reference strains (BC7 and MUCL87) of B. cinerea (Fig. 3). The sequence data of the isolates for GAPDH, HSP60, and RPB2 genes were deposited in GenBank under accession numbers PP806641‒PP806643, PP806644‒PP806646, and PP806647PP806649, respectively.
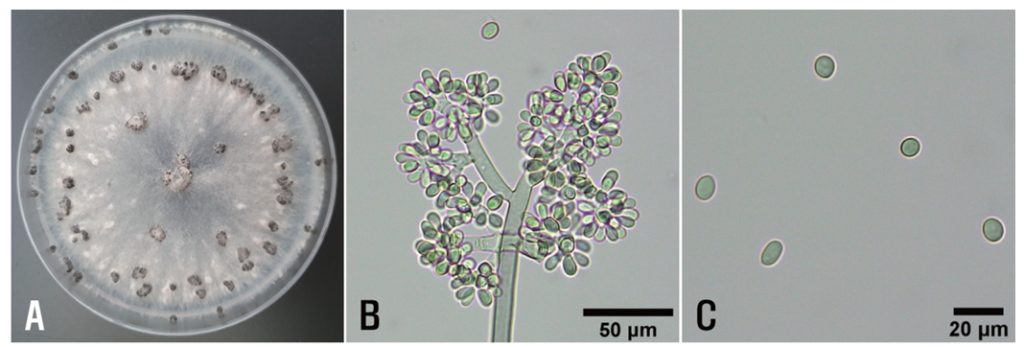
Fig. 2. Cultural and morphological features of Botrytis cinerea isolates from diseased stringy stonecrop plants. A: Colony morphology of isolate SSBC-2405 grown on potato dextrose agar at 20℃ for 21 days. B: Conidiophores and conidia. C: Conidial morphology.
To confirm pathogenicity of the isolates, healthy stringy stonecrop plants were grown in circular plastic pots (height: 13.5 cm; upper diameter: 15 cm; lower diameter: 9 cm) in a vinyl greenhouse. PDA cultures of each isolate were used to prepare conidial suspension (1×106 conidia/mL). Each plant was sprayed with 30 mL of the conidial suspension, while control plants received same quantity of sterile distilled water. All plants were placed in plastic boxes (71.0×53.5×40.5 cm) under 100% relative humidity at 19‒21℃ for 5 days before being transferred to the vinyl greenhouse. Inoculation tests were performed in triplicate. After 10 days, gray mold symptoms were observed on the inoculated plants (Fig. 1C), while no symptoms appeared on the control plants (Fig. 1D). The gray mold symptoms appeared in the pathogenicity tests were similar to those observed in the surveyed vinyl greenhouses. Morphologically identical fungi were reisolated from the lesions.
Gray mold affects a wide range of crops, infecting leaves, stems, flowers, and fruits of over 200 species globally [9]. According to the USDA Fungal Databases [10], Erysiphe spp. and Pythium aphanidermatum (Edson) Fitzp. have been previously reported as pathogens of stringy stonecrop. In Korea, powdery mildew, root and stem rot, and Sclerotinia rot have been reported to occur in stringy stonecrop [11]. However, gray mold of stringy stonecrop has not been previously reported. This is the first report of B. cinerea causing gray mold in stringy stonecrop.
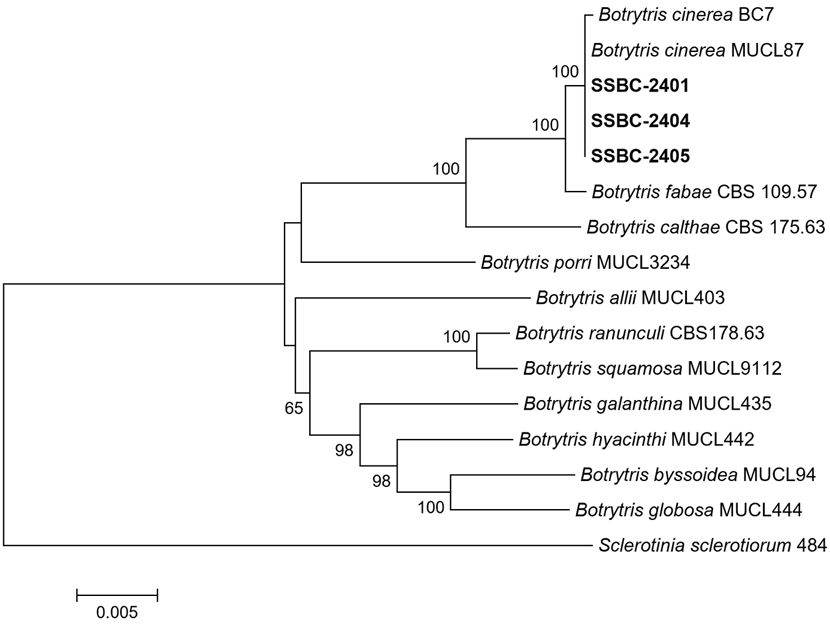
Fig. 3. Phylogenetic tree based on glyceraldehyde-3-phosphate dehydrogenase, heat-shock protein 60, and RNA polymerase II second largest subunit gene regions of the three isolates (SSBC-2401, SSBC-2404, and SSBC-2405) from stringy stonecrop and reference strains of Botrytis species. Sequence data of the reference species were retrieved from the GenBank database of the National Center for Biotechnology Information (NCBI). The phylogenetic tree was constructed using the neighbor-joining method with the maximum composite likelihood model. Bootstrap support values over 50 are shown at the nodes. The scale bar represents the number of nucleotide substitutions per site.
No conflict of interest was reported by the authors.
This study was supported by a research grant (RS-2024-00348961) from the Rural Development Administration, Korea.
1. Plants of the World Online. Sedum sarmentosum Bunge. [Internet]. Kew: Royal Botanic Garden; 2024 [cited 2024 May 1]. Available from https://powo.science.kew.org/.
2. Jiang Z, Wang X, Wang J, Liu C, Pan J. Simultaneous determination of 8 flavonoids in Sedum sarmentosum Bunge from different areas by UHPLC with triple quadrupole MS/MS. Biomed Chromatogr 2019;e4601. [DOI]
3. Ellis MB. Dematiaceous Hyphomycetes. p. 178-9, Kew, Surrey, England: Commonwealth Mycological Institute; 1971. [DOI]
4. Dong L, Liu S, Li J, Tharreau D, Liu P, Tao D, Yang Q. A rapid and simple method for DNApreparation of Magnaporthe oryzae from single rice blast lesions for PCR-based molecular analysis. Plant Pathol J 2022;38:679-84. [DOI]
5. Staats M, van Baarlen P, van Kan JA. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Mol Biol Evol 2005;22:333-46. [DOI]
6. van der Vlugt-Bergmans CJB, Brandwagt BF, van’t Klooster JW, Wagemakers CAM, van Kan JAL. 1993. Genetic variation and segregation of DNA polymorphisms in Botrytis cinerea. Mycol Res 1993;97:1193-200. [DOI]
7. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004;32:1792-7. [DOI]
8. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870-4. [DOI]
9. Williamson B, Tudzynski B, Tudzynski P, van Kan JAL. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 2007;8:561-80. [DOI]
10. USDA Fungal Databases. Sedum sarmentosum Bunge. [Internet]. U.S. Department of Agriculture; 2024 [cited 2024 May 1]. Available from https://fungi.ars.usda.gov/.
11. List of Plant Diseases in Korea. [Internet]. Seoul: Korean Society of Plant Pathology; 2024 [cited 2024 May 1]. Available from http://genebank.rda.go.kr/kplantdisease.do.