Ju-Heon Lee1,2, YoungSoo Kim1, Leonid N. Ten3, Jong-Taek Park1, Dong-Hyuk Lee1, and Hee-Young Jung2,3*
1Apple Research Center, National Institute of Horticultural & Herbal Science, Gunwi 43100, Korea
2Department of Plant Medicine, Kyungpook National University, Daegu 41566, Korea
3Institute of Plant Medicine, Kyungpook National University, Daegu 41566, Korea
*Correspondence to Email: heeyoung@knu.ac.kr, Tel: +82-53-950-5760, Fax: +82-53-950-6758
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 247-255.
https://doi.org/10.4489/kjm.520403
Received on October 04, 2024, Revised on November 20, 2024, Accepted on November 29, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
In this study, ambrosia beetles were collected using insect traps in Gunwi-gun, Daegu-si, South Korea, to investigate symbiotic fungi associated with beetles. Fungi were isolated from the collected beetles. Among the isolates, a strain obtained from Ambrosiodmus rubricollis was designated as ARI-24-A4. Cultural and molecular biological analyses confirmed that ARI24-A4 belongs to Ambrosiella. Key morphological characteristics, including the structure of the conidiophores and the size of aleurioconidia (av. 12.9 μm × 12.5 μm), were examined to accurately identify the Ambrosiella species. A phylogenetic tree was constructed by combining the internal transcribed spacer (ITS) region, translation elongation factor 1-alpha (TEF1-α), and small subunit of nuclear ribosomal RNA (SSU) gene sequences to confirm the phylogenetic position. The strain was verified to share the same phylogenetic position as Ambrosiella roeperi. Therefore, ARI-24-A4 was confirmed to be A. roeperi, a species previously unreported in Korea. It has been recorded as a newly identified species in the country.
Ambrosia beetles, Ambrosiella roeperi, Phylogeny, Symbiotic
Ambrosiella is a fungal genus defined by Harrington [1]. It belongs to the family Ceratocystidaceae within the order Microascales of the class Sordariomycetes. Many species are dispersed by insect vectors or through the frass of ambrosia beetles; some of them act as tree pathogens [2,3]. Currently, 20 Ambrosiella species are recorded in the Mycobank database. Among them, A. xylebori [4], A. hartigii [5], A. beaveri [6], and A. roeperi [7] are closely associated with the mesonotal pouch mycangia of the Xylosandrus complex. These mycangia are specialized beetle structures that store and transport symbiotic fungi into trees.
Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) are major pests that bore tunnels into trees, consequently damaging the xylem and substantially harming forests and orchards [8]. Although they were historically regarded as forest pests [9], recent reports from Europe and the United States have shown that they can severely damage fruit trees such as apples, pears, and stone fruits. In Korea, Xyleborinus saxesenii, Xylosandrus germanus, Ambrosiodmus rubricollis, and Anisandrus apicalis have been identified as major pests infesting apple trees [10]. They cultivate symbiotic fungi in nutrient-poor tree sections, which they use as a food source. As a result, this symbiotic relationship often causes the death of the host tree [11].
Ambrosiella species typically produce phialidic conidiophores characterized by the production of aleurioconidia located singly at the tips of aleurioconidiophores. These spores form short chains, and conidiophores have small collarettes at the apex [1,9]. The sexual state of Ambrosiella remains unknown, and genetic variations within the genus are limited [12]. Additionally, these species have been recovered or reported from five beetle genera [7].
In this study, symbiotic fungi were isolated to investigate the diversity and distribution of symbiotic fungi associated with the four major ambrosia beetle species known to affect apple trees in Korea. Among them, the fungal strain isolated from A. rubricollis was identified through morphological and molecular biological characteristics, and its phylogenetic position was determined.
Ambrosia beetles (A. rubricollis) were collected using a trap at the Apple Research Center in Gunwi-gun, Daegu-si, Korea (36°29′68.9″N, 128°46′56.1″E). Their bodies were surface-sterilized with 70% ethanol and then dried thoroughly for approximately 20 min. Afterward, the insects were dissected by separating the head and thorax from the abdomen. The segments were placed onto potato dextrose agar (PDA; Difco, Detroit, MI, USA) plates and incubated at 25℃ for 3 days. The grown mycelium was transferred to fresh PDA plates and incubated for 8 more days at 25℃. The pure isolated strain was designated as ARI-24-A4 and preserved in 20% glycerol stock at −80℃ for further analysis.
ARI-24-A4 was cultured on PDA at 25℃ for 8 days to assess its cultural and morphological characteristics. Following incubation, the diameter, color, and shape of the fungal colony were recorded. The morphological characteristics, including conidia and conidiophores, were observed through light microscopy (CX-43, Olympus, Japan).
Fungal mycelia were cultured on PDA plates at 25℃ for 5 days and scraped off using a sterile blade to extract genomic DNA. DNA was extracted using a HiGene genomic DNA prep kit (Biofact, Daejeon, Korea) in accordance with the manufacturer’s instructions. Polymerase chain reaction (PCR) was performed targeting the internal transcribed spacer (ITS) regions, the small subunit of nuclear ribosomal RNA (SSU), and the partial sequences of the translation elongation factor 1-alpha (TEF1-α) gene. The ITS regions were amplified using the ITS1F/ITS4 primers [13,14]. The SSU gene was amplified using the NS-1/NS-6 primers [14,15]. The TEF1-α gene was amplified using the EFCF1.5/EFCF6 primer pair [16]. The PCR products were verified on 1% agarose gels and stained with ethidium bromide. The amplified PCR products were purified using an EXOSAP-IT kit (Thermo Fisher Scientific, Waltham, MA, USA) in accordance with the manufacturer’s instructions, and the purified DNA fragments were sequenced by Macrogen Co., Ltd. (Daejeon, Korea). Sequence data were analyzed using SeqMan Lasergene software (DNAStar Inc., Madison, Wisconsin, USA). The ITS regions, SSU, and TEF1-α gene sequences were deposited in GenBank under accession numbers LC835910, LC835912, and LC835914, respectively.
The sequences of Ambrosiella species were obtained from the National Center for Biotechnology Information (NCBI) database (Table 1). These sequences were aligned using Clustal X 2.0 in MEGA 7 (https://www.megasoftware.net/) [17]. The concatenated nucleotide sequences that included the ITS region and partial sequences of the TEF1-α and SSU genes were phylogenetically analyzed using the maximumlikelihood (ML) [18] method. In ML analysis, the nearest neighbor interchange heuristic search method and Kimura’s two-parameter model were used [19], and gaps were excluded from the analysis. The reliability of the ML analysis was indicated by bootstrap values derived from 1,000 replicates.
Table 1. List of species included in the phylogenetic analyses and their corresponding GenBank accession numbers
| Species | Strain | Associated ambrosia beetle | GenBank accession numbers | ||
|---|---|---|---|---|---|
| ITS | TEF1-α | SSU | |||
| Ambrosiella batrae | CBS 139735T | Anisandrus sayi | KR611322 | KT290320 | KR673881 |
| Ambrosiella beaveri | CBS 121750 | Cnestus mutilatus | KF669875 | KT318380 | KR673882 |
| Ambrosiella catenulata | C3913 | Ambrosia beetle | MG950184 | MG944394 | MG950189 |
| Ambrosiella cleistominuta | C3843 | Anisandrus maiche | KX909940 | KX925304 | KX925309 |
| Ambrosiella grosmanniae | CBS 137359T | Xylosandrus germanus | KR611324 | KT318382 | KR673884 |
| Ambrosiella hartigii | CBS 404.82 | Anisandrus dispar | KF669873 | KT318383 | KR673885 |
| Ambrosiella nakashimae | CBS 139739T | Xylosandrus amputatus | KR611323 | KT318381 | KR673883 |
| Ambrosiella remansi | M290 | Remansus mutabilis | KX342068 | KX342072 | KX354426 |
| Ambrosiella roeperi | ARI-24-A4 | Ambrosiodmus rubricollis | LC835910 | LC835914 | LC835912 |
| Ambrosiella roeperi | CBS 135864T | Xylosandrus crassiusculus | KF669871 | KT318384 | KR673886 |
| Ambrosiella xylebori | CBS 110.61T | Xylosandrus compactus | KF669874 | KT318385 | KR673887 |
| Catunica adiposa | CBS 183.86 | N/A | JN604448 | HM569644 | KR673891 |
ITS: internal transcribed spacer; TEF1-α: translation elongation factor 1-alpha; SSU: small subunit of nuclear ribosomal RNA. T: ex-type. The isolated strain is shown in bold.
When cultured on PDA at 25℃ for 8 days, the diameter of colony growth was 65.2–70.2 mm. The front side developed white conidia at the center, appearing predominantly white but changing to olivaceous toward the edges (Fig. 1A). On the back side, the overall color was brown, but it lightened to a pale brown toward the edges (Fig. 1B). As the culture period extended, dark-brown droplets exuded from the mycelium, and a sour smell was released.
Aleurioconidiophores are hyaline, smooth, and cylindrical, with conidia and a collarette forming at the terminal ends of conidiophores (Figs. 1C and 1D). Aleurioconidia are hyaline with visible cell organelles inside. Their cell walls are thick, and the conidia are nearly cylindrical, with an average size of 12.9 µm × 12.5 µm (n = 50). When aleurioconidia detach from aleurioconidiophores, most of them carry along a conidiophore cell (Fig. 1F), and the same collarette type can be observed. While most aleurioconidia appear spherical, collarettes are cylindrical, and the point of attachment to the conidiophore cell is blunt; therefore, these characteristics clearly distinguish the two forms (Figs. 1G and 1H).
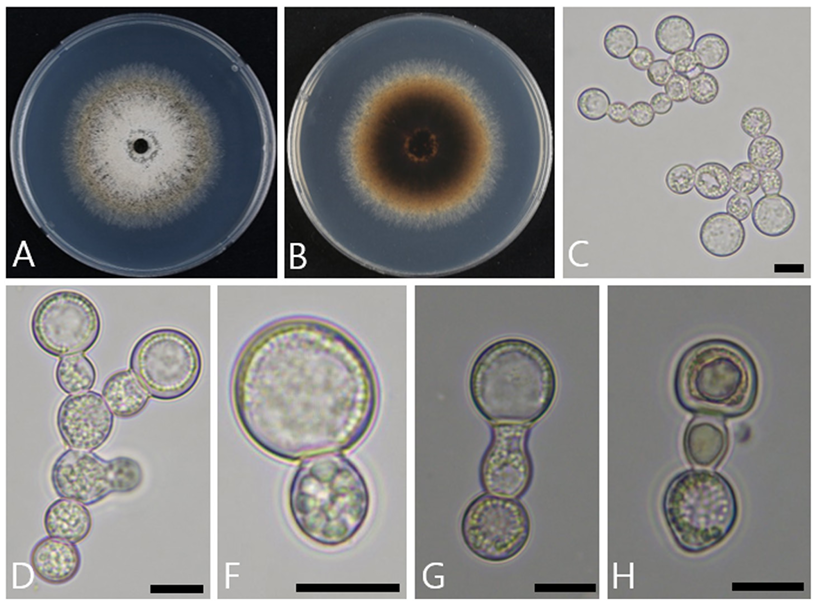
Fig. 1. Cultural and morphological characteristics of Ambrosiella roperi. A, B: Front and reverse view of the colony grown on potato dextrose agar (PDA) for 8 days at 25℃. C, D: Aleurioconidiophores with terminal aleurioconidia. F: Aleurioconidia. G: Aleurioconidiophores with aleurioconidia. H: Aleurioconidiophores with collarettes. Scale bars C–H = 10 μm.
The cultural and morphological characteristics of ARI-24-A4 were consistent with those of A. roeperi. However, ARI-24-A4 clearly differed from its closely related species A. grosmanniae. In terms of cultural characteristics, the growth rate and colony color clearly varied. When cultured on PDA for 8 days, ARI-24-A4 reached a diameter of 65.2–70.4 mm; by comparison, A. grosmanniae grew faster, reaching 67.0–85.0 mm in diameter. In terms of color, ARI-24-A4 displayed a white to olive coloration, whereas A. grosmanniae was gray to dark gray. Therefore, they were clearly distinct from each other. In terms of morphological characteristics, ARI-24-A4 had significantly larger conidia (12.9 µm × 12.5 µm) than A. grosmanniae (7.5–12.0 µm × 7.5–12.0 µm). These differences confirmed that ARI-24-A4 is distinct from A. grosmanniae (Table 2).
Table 2. Comparison of the morphological characteristics of the ARI-24-A4 strain and the reference species Ambrosiella roeperi and A. grosmanniae
| Characteristics | A. roeperia (ARI-24-A4) | A. roeperib | A. grosmanniaeb,c | |
|---|---|---|---|---|
| Colony | Color | Front side: White conidia found in the center, and changes in color to olivaceous toward the periphery Back side: Brown overall, becoming lighter toward the edges | Olivaceous to gray, dark gray, to dark-brown superficial | Gray to dark gray with an abundant aerial mycelium |
| Shape | Colonies on PDA attaining 65.2–70.4 mm diam after 8 days at 25℃ | Colonies on PDA attaining 60.5–70.0 mm diam after 8 days at 25℃ | Colonies on PDA attaining 67.0–85.0 mm diam after 8 days at 25℃ | |
| Aleurio conidiophores | Color | Hyaline | Hyaline to subhyaline | Hyaline |
| Shape | Smooth and cylindrical, with organelles observed within the cells; a single spore or a collarette at the terminal of the conidiophore | Smooth, cylindrical, monilioid, branched, with or without a collarette on the top cell of conidiophores | Smooth, ellipsoidal with or without collarettes on the top cell of conidiophores | |
| Aleurio conidia | Color | Hyaline | Hyaline | Hyaline to subhyaline |
| Shape | Thick-walled, smooth, with organelles observed, almost spherical in shape | Thick-walled, globose to subglobose | Thick-walled, globose single and terminal on aleu rioconidiophores | |
| Size (μm) | (9–)12.9(–16) × (9–)12.5(–16) | (7– )9.0–13.0(–16) × (5– )8.0–12.0(–14) | 7.5–12.0 × 7.5–12.0 |
a Fungal strain studied in this paper; b Source of description [23]; c Sources of description [22].
The partial nucleotide sequences of the ITS regions, TEF1-α, and SSU genes to analyze the molecular and phylogenetic relationships of ARI-24-A4, and their lengths were 591, 1,095, and 1,299 bp, respectively. BLAST searches were conducted to compare these sequences with those registered in the NCBI database. For the ITS region, ARI-24-A4 exhibited the highest similarity of 100.0% with A. roeperi C2448, 97.5% similarity with A. catenulata W20-44-06, 96.3% with A. grosmanniae CBS 137359, and 95.7% with A. xylebori CBS 110.61. Based on the TEF1-α gene sequence, ARI-24-A4 showed a 100.0% similarity with A. roeperi CBS 135864, 95.2% with A. xylebori AFTOL-ID 1285, 94.8% with A. grosmanniae 1002HHS1, and 93.7% with A. xylebori C1650. For the ITS region, the species with the highest similarity of 100.0% was A. roeperi. For the SSU gene sequence, ARI24-A4 exhibited 99.9% similarity with A. batrae CBS 139735, 99.8% with A. xylebori CBS 110.61, 99.8% with A. roeperi CBS 135864, and 99.8% with A. catenulata C3913.
For the ITS region and TEF1-α gene sequences, A. roeperi was identified as the most closely related species with a 100.0% similarity. However, for the SSU gene sequence, no clear distinction was observed as the similarities were 99.9–99.8% for A. batrae, A. xylebori, and A. roeperi. Therefore, multilocus sequence analysis was performed by concatenating the ITS, TEF1-α, and SSU sequences, and a phylogenetic tree was constructed using the ML method to determine the phylogenetic position of ARI24-A4. The phylogenetic tree revealed that ARI-24-A4 formed a clade with A. roeperi, indicating a specieslevel relationship with A. roeperi (Fig. 2). Additionally, the phylogenetic tree clearly distinguished ARI24-A4 from A. grosmanniae, A. batrae, and A. xylebori, which exhibited a high SSU sequence similarity. The topology of the ML tree indicated that A. grosmanniae could be the second close phylogenetic relative of the isolate. The mycological characteristics and phylogenetic position of ARI-24-A4 confirmed that it is indeed identical to A. roeperi at the species level. This study is the first to report this fungal species in Korea.
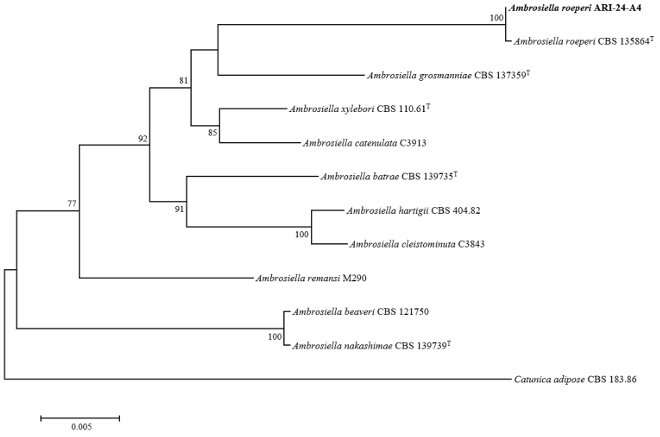
Fig. 2. Maximum-likelihood phylogenetic tree of ARI-24-A4 based on the combined sequences (ITS+TEF1-α+SSU), showing the phylogenetic position of the ARI-24-A4 strain among Ambrosiella species. Bootstrap values (based on 1,000 replications) greater than 70% are shown at branch points. The isolated strain is shown in bold. Catunica adiposa CBS 183.86 was used as an outgroup. Bar, 0.005 substitutions per nucleotide position. ʻT’ indicates the type strain. ITS: internal transcribed spacer; TEF1-α: translation elongation factor 1-alpha; SSU: small subunit of nuclear ribosomal RNA.
Ambrosiella species have been reported as symbiotic fungi associated with ambrosia beetles [20] by participating in the life cycle of beetles. These fungi have been found in ambrosia beetles with relatively large and complex mycangia (specialized structures for storing fungi) and have been reported from five beetle genera [7]. Studies on the mycangia of ambrosia beetles have shown that gland cells secrete substances into or near the mycangium, thereby supporting the growth of fungal symbionts [21]. furthermore, spores overflowing from the mycangium inoculate the galleries during construction. Female Xylosandrus, Anisandrus, and Cnestus species within the tribe Xyleborini have large mesonotal mycangia, which are internal structures closely associated with Ambrosiella fungi. A. hartigii, A. beaveri, and A. roeperi are species associated with the mesonotal pouch mycangia of the Xylosandrus complex and known as key symbiotic fungi of ambrosia beetles. A. ferruginea and A. trypodendri are related to the prothoracic pleural mycangia of Trypodendron species [1,20].
In this study, ARI-24-A4 was discovered in A. rubricollis. BLAST searches using the sequences of the ITS regions, TEF1-α, and SSU genes confirmed that it represents a novel A. roeperi strain, and its close neighbor is A. grosmanniae. In previous studies, several molecular markers were used to identify Ambrosiella species. In 2014, Harrington utilized the large-subunit rDNA (LSU) single-gene sequence to identify five species: A. beaveri, A. ferruginea, A. hartigii, A. roeperi, and A. xylebori [7]. The phylogenetic positions of these species were confirmed by the concatenated sequences of the TEF1-α and SSU genes [22]. Later, the concatenated sequences of the ITS regions, TEF1-α, and RNA polymerase II subunit 1 (RPB1) genes were used to classify the novel species A. catenulata [23]. Despite the morphological differences between A. nakashimae and A. beaveri, this study revealed that their molecular markers were insufficient to distinguish between them. In our study, we used a different combination of three molecular markers, namely, the ITS regions, TEF1-α, and SSU genes, which were available for a larger number of Ambrosiella species than the RPB1 sequences. The results showed that ARI-24-A4 occupied a phylogenetic position alongside A. roeperi CBS 135864T, and both strains were clearly different from other Ambrosiella species. The combination of the molecular markers used in this study was sufficient to distinguish A. roeperi and various other Ambrosiella species; however, similar to the case of the molecular markers in previous studies, such a combination did not provide a clear differentiation between A. beaveri and A. nakashimae. Therefore, additional specific genetic markers are needed to accurately differentiate Ambrosiella species.
ARI-24-A4, which was isolated from A. rubricollis in Korea, is an unrecorded species in Korea. Morphological and molecular analyses confirmed that it is the same as A. roeperi, suggesting that Ambrosiella species may be associated with Ambrosiodmus beetles. Thus, this study expands our understanding of the distribution of Ambrosiella and emphasizes the need for further investigations into the correlation, distribution, and biological roles of symbiotic fungi and ambrosia beetles.
The authors declare that they have no potential conflicts of interest.
This work was performed with the support of the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01718404)” funded by the Rural Development Administration, Republic of Korea.
1. Harrington TC, Aghayeva DN, Fraedrich SW. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 2010;111:337-61. [DOI]
2. Harrington TC. The genus Ceratocystis. Where does the oak wilt fungus fit? In: Billings RF, Appel DN, editors. Proceedings of the 2nd National Oak Wilt Symposium. Austin, Texas: Texas Forest Service Publication 166; 2009. p. 21-35.
3. Harrington TC. Ceratocystis diseases. In: Gonthier P, Nicolotti G, editors. Infectious Forest Diseases. Wallingford, UK: CABI International; 2013. p. 230-55. [DOI]
4. Brader L. Étude de la relation entre le scolyte des rameaux du caféier, Xyleborus compactus Eichh. (X. morstatti Hag.), et sa plante-hôte [dissertation]. Wageningen: Wageningen University; 1964.
5. Batra LR. Ambrosia fungi-a taxonomic revision, and nutritional studies of some species. Mycologia 1967;59:976-1017. [DOI]
6. Six DL, Stone WD, de Beer ZW, Woolfolk SW. Ambrosiella beaveri, sp. nov., associated with an exotic ambrosia beetle, Xylosandrus mutilatus (Coleoptera: Curculionidae, Scolytinae), in Mississippi, USA. Antonie van Leeuwenhoek 2009;96:17-29. [DOI]
7. Harrington TC, McNew D, Mayers C, Fraedrich SW, Reed SE. Ambrosiella roeperi sp. nov. is the mycangial symbiont of the granulate ambrosia beetle, Xylosandrus crassiusculus. Mycologia 2014;106:835-45. [DOI]
8. VanDerLaan NR, Ginzel MD. The capacity of conophthorin to enhance the attraction of two Xylosandrus species (Coleoptera: Curculionidae: Scolytinae) to ethanol and the efficacy of vervenone as a deterrent. Agric For Entomol 2013;15:391-7. [DOI]
9. Kwon TS, Lee BW, Park SY, Byun BK, Park SW, Lee CM. Diversity and abundance of bark beetles (Coleoptera, Curculionidae, Scolytinae and Platypodinae) in deadwoods of Quercus serrata and Carpinus laxiflora. Kor J Appl Entomol 2011;50:353-62. [DOI]
10. Kim JW, Kim SJ, Lee SY, Nam JC, Lee DH, Choi KH. Seasonal occurrence and pesticide control of ambrosia beetles (Curculionidae: Scolytinae) in apple orchard. Korean J Pestic Sci 2018;22:284-91. [DOI]
11. Werle CT, Chong JH, Sampson BJ, Reding ME, Adamczyk JJ. Seasonal and spatial dispersal patterns of select ambrosia beetles (Coleoptera: Curculionidae) from forest habitats into production nurseries. Fla Entomol 2015;98:884-91. [DOI]
12. van de Peppel LJ, Aanen DK, Biedermann PH. Low intraspecific genetic diversity indicates asexuality and vertical transmission in the fungal cultivars of ambrosia beetles. Fungal Ecol 2018;32:57-64. [DOI]
13. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8. [DOI]
14. White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press, Inc.; 1990. p. 315-22. [DOI]
15. Vilgalys, R. Conserved primer sequences for PCR amplification and sequencing from nuclear ribosomal RNA [Internet]. Medicine: Duke University; 2018 [cited 2024 Nov 20]. Available from https://sites.duke.edu/vilgalyslab/rdna_primers_for_fungi/.
16. Oliveira LS, Harrington TC, Ferreira MA, Damacena MB, Al-Sadi AM, Al-Mahmooli IH, Alfenas AC. Species or genotypes? Reassessment of four recently described species of the Ceratocystis wilt pathogen, Ceratocystis fimbriata, on Mangifera indica. Phytopathology 2015;105:1229-44. [DOI]
17. Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870-4. [DOI]
18. Fitch WM. Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool 1971;20:406-16. [DOI]
19. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Mol Biol Evol 1980;16:111-20. [DOI]
20. von Arx JA, Hennebert GL. Deux champignons ambrosia. Mycopathol Mycol Appl 1965;25:309-15. [DOI]
21. Schneider I, Rudinsky JA. Anatomical and histological changes in internal organs of adult Trypodendron lineatum, Gnathotrichus retusus, and G. sulcatus (Coleoptera: Scolytidae). Ann Entomol Soc Am 1969;62:995-1003. [DOI]
22. Mayers CG, McNew DL, Harrington TC, Roeper RA, Fraedrich SW, Biedermann PH, Castrillo LA, Reed SE. Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fungal Biol 2015;119:1075-92. [DOI]
23. Lin YT, Shih HH, Hulcr J, Lin CS, Lu SS, Chen CY. Ambrosiella in Taiwan including one new species. Mycoscience 2017;58:242-52. [DOI]