Bora Nam1† , Joon-Ho Choi2† , Seong-Jin Lee3, Hyeon-Dong Shin4 , and Young-Joon Choi1,2*
1Center for Convergent Agrobioengineering, Kunsan National University, Gunsan 54150, Korea
2Department of Biological Science, Kunsan National University, Gunsan 54150, Korea
3Department of Plant Quarantine, Animal and Plant Quarantine Agency, Gimcheon 39660, Korea
4Division of Environmental Science and Ecological Engineering, Korea University, Seoul 02841, Korea
*Correspondence to yjchoi@kunsan.ac.kr
†The first two authors contributed equally to this study.
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 277-282.
https://doi.org/10.4489/kjm.520406
Received on November 20, 2024, Revised on December 05, 2024, Accepted on December 05, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Crassocephalum crepidioides is a flowering plant in the Asteraceae family that is native to tropical Africa but has become a significant weed in several Asian regions, including Korea. In October 2022, angular leaf spots were observed on C. crepidioides that coalesced into larger lesions with visible pycnidial conidiomata and conidial tendrils under moist conditions. Morphological and molecular analyses identified the causative pathogen as Septoria gynurae. This is the first report of S. gynurae and a fungal disease affecting C. crepidioides in Korea. This study provides the first molecular phylogenetic information on this species and reveals its close relationship with other Septoria species that infect Asteraceae. Septoria gynurae is a potential biocontrol agent; however, further studies are needed to confirm its host specificity and broader effects.
fireweed, multi-loci phylogeny, redflower ragleaf
Crassocephalum crepidioides (Benth.) S. Moore, also known as ebolo, redflower ragleaf, or fireweed, is a flowering plant belonging to the Asteraceae family. It was previously classified as Gynura crepidioides Benth., Gynura sarcobensis DC., and Senecio diversifolius A. Rich [1]. Native to tropical Africa, this plant has been introduced to many Asian countries [1], where it has become a problematic invasive species [2]. In Korea, C. crepidioides was unintentionally introduced around 1980 and has since spread extensively, particularly in southern regions [3]. Now, it is recognized as one of the most dominant and threatening exotic weeds in Korean crop fields [4,5]. It can outcompete both native and crop plants due to its adaptability to diverse environments.
In October 2022, our plant disease survey revealed angular leaf spots on the leaves of C. crepidioides in Sunchang (35°27’54″N 127°12’30″E), Korea. Leaf spots were angular and vein-limited but coalesced to form larger, irregular lesions (Fig. 1A and 1B). Under wet conditions, the pycnidial conidiomata and conidial tendrils were easily visible on the upper part of the leaf lesions (Fig. 1C). To obtain a pure isolate, the conidial tendril from the lesions was placed in sterile water, and the conidial suspension was streaked onto 2% water agar (WA). After 4 d, a hyphal tip protruding from the developing colony was transferred onto potato dextrose agar (PDA). The 3-week-old colonies incubated at 25 °C on PDA were 10–15 mm in diameter, surface folded, cerebriform, olivaceous gray, and covered by white mycelium with an irregular margin (Fig. 1H). Voucher specimens were housed in the Korea University Herbarium (KUS-F33481) and a representative culture was deposited in the Korea Agricultural Culture Collection (Acc. No. KACC 410471).
The morphological characteristics of the causal pathogen were observed under an M205C stereomicroscope (Leica, Wetzlar, Germany) equipped with a Dhyana 400DC camera (Tucsen, Fuzhou, China) and a DIC microscope (Axio Imager M2 AX10, Carl Zeiss, Jena, Germany) with an Axio Cam 512 camera (Carl Zeiss). Conidiomata were pycnidial, amphigenous but mostly epiphyllous, 52–80 μm in diameter, and ostiolate with openings of 20–30 μm in diameter (Figs. 1D and 1E). Conidia were hyaline, rod-shaped to filiform, straight to mildly curved, guttulate, 1–3-septate, and 26 to 38 × 2 to 3 μm, with acute apex and sub-truncate base (Figs. 1F and 1G). The morphological characteristics of the fungus were closest to those of Septoria gynurae Katsuki on Gynura bicolor Roxb. ex Willd in Japan [6], although there is a minor difference in the conidial morphology, with 1-septate and narrower conidia ranging from 22 to 48 × 1 to 1.8 μm. However, the conidial characters of the Korean specimen are well in line with those of S. gynurae on C. crepidioides in China, which has 2–3 septa and measures 2–3 μm wide [7].
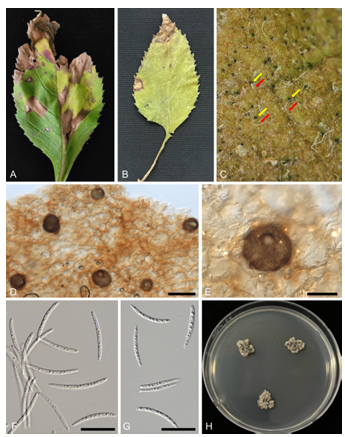
Fig. 1. Leaf spot disease caused by Septoria gynurae on Crassocephalum crepidioides. A and B: General view of infected fresh (A) and dried (B) leaves. C: Close-up view of a leaf lesion with blackish pycnidial conidiomata (yellow arrow) and hyaline conidial tendrils (red arrow). D and E: Pycnidial conidiomata with ostioles on the leaf surface. F and G: Conidia. H: 3-week-old colonies of S. gynurae growing on potato dextrose agar (PDA). Scale bars: D = 100 μm, E = 50 μm, F and G = 20 μm.
To verify this identity, G-DNA was extracted from a herbarium specimen (KACC 410471) using the MagListo 5M Plant Genomic DNA Extraction Kit (Bioneer, Daejeon, Korea). PCR amplifications were performed using primer pairs: ITS4/ITS5 [8] for the internal transcribed spacer (ITS), ACT-512F/ACT783R [9] for actin (actA), CAL-235F [10]/CAL2Rd [11] for Calmodulin (Cal), T1 [12]/b-sandy-R [13] for β-tubulin (Btub), and fRPB2-5F [14]/fRPB2-414R [15] for RNA polymerase II second largest subunit (RPB2). The amplified PCR products were analyzed on a 1.5% agarose gel by electrophoresis, purified using an AccuPrep PCR Purification Kit (Bioneer, Daejeon, Korea), and sequenced by Macrogen (Daejeon, Korea). The obtained sequences were submitted to GenBank under the accession numbers PQ187655, PQ202454, PQ202455, PQ202456, and PQ202457. Because this study initially provided sequences of Septoria gynurae, there was no reference sequence for this species in GenBank. A Blastn search showed 96.65% similarity with sequences of Septoria dearnessii (MH865052) and S. mazi (MH865129) for ITS, 91.87% identity to S. lactucae (KF253743) for actA, 96.06% similarity to S. lactucae (KF254091) for Cal, 91.23% similarity to S. lactucae (KF252911) for Btub, 97.67% similarity to S. chelidonii (KF252436) for RPB2. Multilocus phylogenetic analysis was performed using combined ITS, actA, Cal, Btub, and RPB2 datasets. The sequences of the individual markers were concatenated in SequenceMatrix v1.7.8 [16] and a phylogenetic tree was reconstructed for minimum evolution (ME) inferences in MEGA version 11 [17]. ME analyses were performed using the Tamura-Nei model with 1,000 bootstrap replicates (BS). In the phylogenetic tree reconstructed using the multi-loci sequences (Fig. 2), the Korean isolate was placed within clade 3 among the three clades (3 –5) of Septoria sensu stricto [18,19] and further formed a wellsupported group with S. lactucae ex Lactuca spp., suggesting the phylogenetic affinity of the two species affecting Asteraceae.
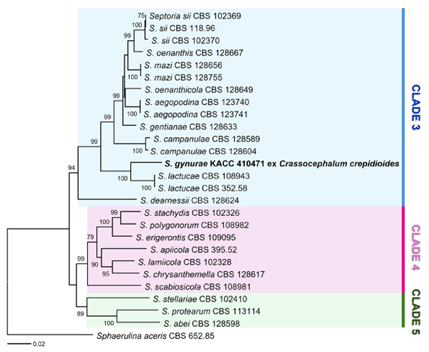
Fig. 2. Minimum evolution tree of Septoria species based on the internal transcribed spacer (ITS), actin (actA), calmodulin (Cal), β-tubulin (Btub), and RNA polymerase II second largest subunit (RPB2). Bootstrapping support values over 70% are given above the branches. The Korean sample affecting Crassocephalum crepidioides is shown in bold. The scale bar equals the number of nucleotide substitutions per site.
The genus Septoria (Mycosphaerellaceae) encompasses many notorious plant pathogens that affect crops and weeds, leading to considerable economic and management challenges, e.g., S. lycopersici on tomatoes (Solanum lycopersicum), S. passifloricola on Passion Fruit (Passiflora edulis), S. lactucae on lettuce (Apium graveolens) [20–23]. They typically cause leaf spots characterized by necrotic lesions with well-defined borders and produce conidia within pycnidia or acervuli, which facilitate the spread of the infection to healthy plant tissues. Septoria species are characterized and identified based on their size, shape, septa, and pycnidia morphology [24–26]. Accurate identification and classification of Septoria species often rely on molecular techniques, such as DNA sequencing, because of the morphological similarities between species [18,19]. Similarly, based on the morphological features and sequencing data, the pathogen affecting C. crepidioides in Korea was identified as S. gynurae.
To our knowledge, this is the first report of S. gynurae as a fungal disease affecting C. crepidioides in Korea. Previously, the association between C. crepidioides and S. gynurae was reported only in China [7,27]. As the infection causes leaf spots and blight, leading to early leaf death, S. gynurae can be used as a biocontrol agent against this troublesome weed. However, although this fungus has only been recorded on C. crepidioides, suggesting high host specificity, further research is needed to accurately determine its host range.
The authors declare that there are no conflicts of interest.
This study was funded by the Plant Quarantine Technology Center of the Animal and Plant Quarantine Agency (grant number PQ20241B012).
1. Belcher RO. The typification of Crassocephalum Moench and Gynura Cass. Kew Bull 1955;10:455-65. [DOI]
2. Chen GQ, Guo SL, Huang QS. Invasiveness evaluation of fireweed (Crassocephalum crepidioides) based on its seed germination features. Weed Biol Manag 2009;9:123-8. [DOI]
3. Ryu TB, Kim JW, Lee SE. The exotic flora of Korea: actual list of neophytes and their ecological characteristics. Korean J Environ Ecol 2017;31:365-80. [DOI]
4. Song HG, Kim CS, Lee J, Seo HA, Lee IY. Occurrence of weed flora in Codonopsis lanceolata upland fields of Gangwon-Hoengseong and Jeju areas in Korea. Weed Turf Sci 2015;4:176-87. [DOI]
5. Oh SM, Moon BC, Kim CS, Lee IY. Distribution of exotic weeds in agricultural fields of the Gyeonggi, Gangwon and Jeju areas in Korea. Korean J Weed Sci 2004;24:138-48.
6. Katsuki S. Notes on parasitic fungi of Yaku island. J Jap Bot 1953;28:279-88.
7. Bai J. Flora Fungorum Sinicorum, Vol. 17, Sphaeropsidales: Ascochyta, Septoria. Beijing: Science Press; 2003.
8. White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, et al., editors. PCR protocols: a guide to methods and applications. Vol. 18. New York: Academic Press Inc; 1990. p. 315-22. [DOI]
9. Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia 1999;91:553-6. [DOI]
10. Quaedvlieg W, Groenewald J, de Jesús Yáñez-Morales M, Crous PW. DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia 2012;29:101-15. [DOI]
11. Groenewald J, Nakashima C, Nishikawa J, Shin HD, Park JH, Jama AN, Groenewald M, Braun U, Crous PW. Species concepts in Cercospora: spotting the weeds among the roses. Stud Mycol 2013;75:115-70. [DOI]
12. O’Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenetics Evol 1997;7:103-16. [DOI]
13. Stukenbrock EH, Quaedvlieg W, Javan-Nikhah M, Zala M, Crous PW, McDonald BA. Zymoseptoria ardabiliae and Z. pseudotritici, two progenitor species of the Septoria tritici leaf blotch fungus Z. tritici (synonym: Mycosphaerella graminicola). Mycologia 2012;104:1397-407. [DOI]
14. Liu YJ, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 1999;16:1799-808. [DOI]
15. Quaedvlieg W, Kema GHJ, Groenewald JZ, Verkley GJM, Seifbarghi S, Ravazi M, Gohari AM, Mehrabi R, Crous PW. Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 2011;26:57-69. [DOI]
16. Vaidya G, Lohman DJ, Meier R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011;27:171-80. [DOI]
17. Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 2021;38:3022-7. [DOI]
18. Quaedvlieg W, Verkley GJM, Shin HD, Barreto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW. Sizing up Septoria. Stud Mycol 2013;75:307-90. [DOI]
19. Verkley GJM, Quaedvlieg W, Shin HD, Crous PW. A new approach to species delimitation in Septoria. Stud Mycol 2013;75:213-305. [DOI]
20. Cabral CS, Fonseca MEN, Boiteux LS, Barboza EA, Veloso JS, Lourenço Jr V, Reis A. Phenotypic and genetic variability of fungal isolates associated with the Septoria leaf spot disease of lettuce (Lactuca sativa) in Brazil. J Plant Dis Prot 2022;129:53-62. [DOI]
21. Dai YL, Wang CC, Lin HL, Wang CL. First report of Septoria blotch of passion fruit caused by Septoria passifloricola in Taiwan. Plant Dis 2020;105:700. [DOI]
22. Dhangar NV, Choudhury D. Septoria leaf spot cause by Septoria lycopersici on tomato: a review. Int J Plant Pathol Microbiol 2022;2:55-9.
23. Das S, Pattanayak S, Bhargavi B. Over view of Septoria diseases on different crops and its management. Int J Environ Agric Biotech 2020;13:351-70. [DOI]
24. Jørstad I. Septoria and septoroid fungi on dicotyleones in Norway. Oslo: Oslo University Press; 1965.
25. Jørstad I. Septoria and septorioid fungi on Gramineae in Norway. Oslo: Oslo University Press; 1967.
26. Sutton BC. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Kew: Commonwealth Mycological Institute; 1980.
27. Farr DF, Rossman AY. Fungal Databases, Syst. Mycol [Internet]. Washington DC: US Department of Agriculture, Agricultural Research Service; 2024 [cited 2024 Nov 19]. Available from https://nt.ars-grin.gov/fungaldatabases/.0