Yun-Jeong Kim, Jae-Eui Cha, Eun-Ju Kim, Ji-Won Kim, and Ahn-Heum Eom*
Department of Biology Education, Korea National University of Education, Cheongju 28173, Korea
*Correspondence to eomah@knue.ac.kr
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 301-309.
https://doi.org/10.4489/kjm.520409
Received on November 25, 2024, Revised on December 10, 2024, Accepted on December 11, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Leaves of Zanthoxylum piperitum (L.) DC. and Juniperus rigida Siebold & Zucc. were collected from Mungyeong-si, Gyeongsangbuk-do, Korea, to isolate the endophytic fungi. The leaves were surface-sterilized and the morphological characteristics of the isolated strains were analyzed. The internal transcribed spacer (ITS), large subunit rDNA (LSU), and β-tubulin (TUB) regions were amplified for conducting molecular phylogenetic analysis. Allocryptovalsa sichuanensis and Beltrania pseudorhombica, both belonging to the order Xylariales, were identified. To the best of our knowledge, this study is the first report of these species in Korea. The morphological and molecular characteristics of the isolated strains are detailed in this study. These findings enhance our understanding of the endophyte diversity and ecology in Korea.
Allocryptovalsa sichuanensis, Beltrania pseudorhombica, Endophytic fungi, Xylariales
Endophytic fungi colonize plants without exhibiting visible symptoms [1], and are known to exist in all plant species [2]. These fungi exhibit high diversity and the number of endophytic species associated with a single plant species is often estimated to be hundreds [3]. The endophytes primarily consist of latent saprophytes and mutualistic species. Some are generalists capable of colonizing a wide range of hosts, whereas others are specialists capable of colonizing few or specific hosts. The diversity of endophytic fungi is influenced by geographic variations [4], climatic characteristics [5,6], and the age and tissue of the host plant [7]. Endophytic fungi are increasingly recognized for their roles in plant pathology and applications in the production of secondary metabolites, highlighting the importance of studying their diversity [8,9].
The order Xylariales is a large group of fungi, encompassing over 92 genera and 795 species, with unitunicate asci [10]. Taxa within Xylariales are generally characterized by well-developed stromata, perithecial ascomata with thick walls, unitunicate asci containing eight ascospores, and the presence of a J+ apical apparatus in the asci [11,12]. Paraphyses are freely arranged at the tips and develop from the hymenial layer. Ascospores typically contain pigments and may possess germ pores, germ slits, transverse septa, or mucilaginous sheaths. The anamorphs of Xylariales are typically hyphomycetous, producing holoblastic conidia [13,14]. In addition to their morphological diversity, Xylariales species occupy various ecological niches and function as plant pathogens, saprophytes, and endophytes. Notably, certain endophytic fungi within this order exhibit strong antagonistic effects against fungal pathogens, indicating their potential for use in biological control [15]. Furthermore, Xylariales are prolific producers of secondary metabolites, including volatile and nonvolatile compounds, which have applications in medicine and agriculture.
Despite the extensive diversity of endophytic fungi globally, endophytic fungal communities in Korea remain underexplored [16]. Identifying and characterizing new species within the order Xylariales can provide valuable insights into their ecological roles and potential applications in biotechnology and agriculture. In the present study, leaves of Zanthoxylum piperitum (L.) DC. and Juniperus rigida Siebold & Zucc were collected from the Mungyeong-si, Gyeongsangbuk-do, Korea. Two previously unknown endophytic fungal species were successfully isolated from these samples. We aimed to explore the morphological characteristics of two endophytic fungi belonging to the order Xylariales and conduct molecular phylogenetic analyses.
Leaves of Z. piperitum were collected on September 22, 2023, and leaves of J. rigida Siebold & Zucc. were collected on March 29, 2024, from Mungyeong-si, Gyeongsangbuk-do, Korea, after ensuring that the leaves were free from visible disease symptoms. The leaves were washed under running water, surfacesterilized in 35% H2 O2 for 1 min, followed by sterilization with 70% ethanol for 30 s. Subsequently, the leaves were cut into 1 cm × 0.5 cm pieces and placed on potato dextrose agar medium (PDA; Difco Lab., Detroit, USA). The plates were incubated at 25℃ for 7 d to allow the growth of endophytic fungi. Once hyphal growth from the interior of the leaves was observed, the fungi were subcultured on the PDA medium to obtain pure isolates [17].
Pure isolates were cultured on PDA and malt extract agar (MEA) at 25℃ for 7 d to assess macroscopic morphological characteristics. Isolates were inoculated onto sterilized pine needles and placed on 2% water agar medium (PNA) to induce sporulation [18]. The microscopic structures were examined using slide cultures [19] and optical microscopy (Axio Imager A2; Carl Zeiss, Oberkochen, Germany). Genomic DNA was extracted from the mycelia using the HiGene Genomic DNA Prep Kit (BioFACT, Daejeon, Korea), following the manufacturer’s protocol. For molecular identification and phylogenetic analysis, specific DNA regions were amplified using polymerase chain reaction (PCR). The internal transcribed spacer (ITS) region was amplified using ITS1F/ITS4 primers [20], the large subunit ribosomal DNA (LSU) region was amplified using LR0R/LR7 primers [21], and the β-tubulin (TUB) region was amplified using Bt2a/Bt2b primers [22]. The PCR products were subjected to agarose gel electrophoresis to confirm the size of the amplified DNA fragments, followed by DNA sequencing (SolGent, Daejeon, Korea). DNA sequences were searched against the National Center for Biotechnology Information (NCBI) database using the Basic Local Alignment Search Tool (BLAST). Phylogenetic trees were constructed using the neighbor-joining (NJ) method in MEGA11 [23], and bootstrap analysis was performed 1,000 times to validate the trees. Information on the unrecorded strains was deposited at the National Institute of Biological Resources (NIBR), and the DNA sequences were submitted to NCBI.
The ITS region analysis of KNUE24N045 showed a 99.63% match with A. sichuanensis HKAS 107017 (NR_175673), and the TUB region showed a 100% match with A. sichuanensis HKAS 107017 (MW775592). Phylogenetic analysis using combined ITS and TUB sequences and the NJ method placed KNUE24N045 in a clade with A. sichuanensis HKAS 107017 (Fig. 1). The ITS region analysis of KNUE23P958 showed a 99.65% match with B. pseudorhombica CBS123380 (NR 148074) and a 99.63% match with B. pseudorhombica CBS123380 (NG 058667). Phylogenetic analysis using combined ITS and LSU sequences and the NJ method placed strain KNUE23P958 in a clade with B. pseudorhombica CBS138003 (Fig. 2).
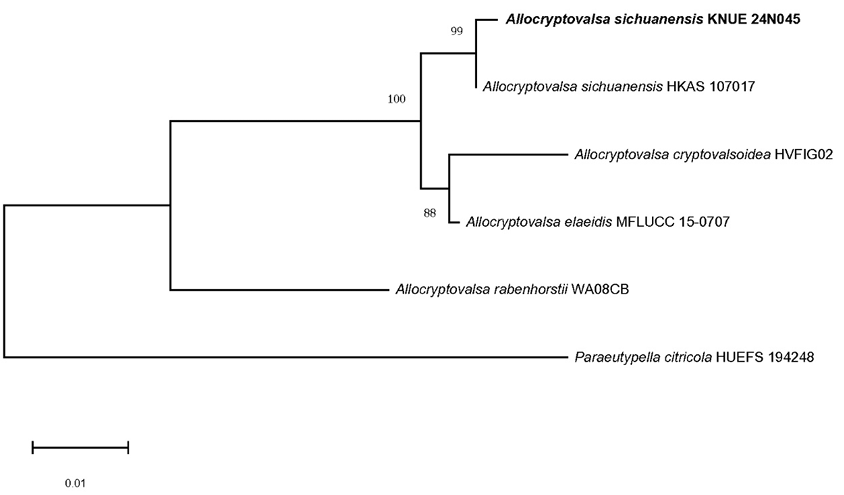
Fig. 1. Neighbor-joining (NJ) phylogenetic tree of Allocryptovalsa sichuanensis KNUE 24N045 based on concatenated sequences of the internal transcribed spacer (ITS) and β-tubulin (TUB) regions. Paraeutypella citricola was used as an outgroup. Numbers on the nodes represent bootstrap values of > 50% (1,000 replicates). Scale bar, number of nucleotide substitutions per site.

Fig. 2. Neighbor-joining (NJ) phylogenetic tree of Beltrania pseudorhombica KNUE 23P958 based on concatenated sequences of the internal transcribed spacer (ITS) and large subunit ribosomal DNA (LSU) regions. Apiospora kogelbergensis was used as an outgroup. Numbers on the nodes represent bootstrap values of > 50% (1,000 replicates). Scale bar, number of nucleotide substitutions per site.
Allocryptovalsa sichuanensis Samarak, Jian K. Liu & K. D. Hyde, Fungal Diversity 112: 30 (2022) [MB#558717] (Fig. 3).
Morphological Characteristics: After culturing on PDA and MEA at 25℃ for 7 d, the macroscopic features were assessed. The colony diameters ranged 68–77 mm on PDA, grew circularly and flat. The texture was soft and cotton-like, with colors ranging from light grey to white on the front and light pink on the reverse, darkening from the edges towards the center. Colonies were 68–70 mm in diameter and circular with broadly spreading soft mycelia when cultured on MEA. The front was light grey, and the reverse was yellow-brown, darkening from the edges to the center.
The asci were thin-walled and unitunicate, with clavate to cylindrical clavate shapes, and apically rounded or truncate ends. They were long, pedicellate, transparent, multinucleate, and occasionally contained granules. Some asci contained ascospores and were polysporous. The ascospores were long, ellipsoidal to allantoid, slightly curved, aseptate, crowded, with smooth walls, and ranged in color from hyaline to yellowbrown. The size of ascospores was 7.86 (6.88–9.27) × 2.61 (2.16–3.26) μm (n=20).
Specimen Examined: Mungyeong-si, Gyeongsangbuk-do, Korea, 36° 39′ 43.312″ N, 128° 10′ 33.596″ E, March 29, 2024, A. sichuanensis, isolated from leaf of J. rigida Siebold & Zucc., strain KNUE 24N045, NIBRFGC000512621, GenBank No. PQ611149 (ITS), and PQ619866 (TUB).
Notes: The genus Allocryptovalsa was introduced in 2017 and is characterized by submerged perithecia, multispored asci, and allantoid ascospores [24]. Members of the Diatrypacea family, to which this genus belongs, are distributed globally and are commonly known as endophytes in angiosperms. They have also been identified as endophytes of gymnosperms and have been reported to cause various plant diseases [25]. A. sichuanensis was first reported in 2022, isolated from dead branches of deciduous trees in the Yuan’an region of China [26]. Clavate to cylindrical clavate, thin-walled asci, and light-yellow ascospores were observed on PNA, and the morphological characteristics of KNUE 24N045 were consistent with those of A. sichuanensis HKAS107017 [26] (Table 1).
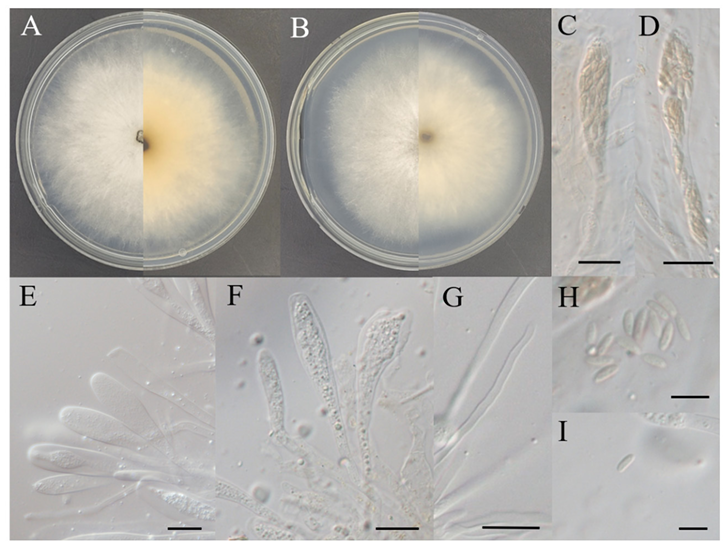
Fig. 3. Morphology of Allocryptovalsa sichuanensis KNUE 24N045. A: Colony grown for 7 d on potato dextrose agar (PDA), B: Colony grown for 7 d on malt extract agar (MEA), C–F: Asci, G: Paraphyses, H, I: Ascospores. Scale bars: C–G, 20 μm; H and I, 10 μm
Table 1. Morphology of strains KNUE 24N045 and HKAS 107017 of Allocryptovalsa sichuanensis
| Characteristics | A. sichuanensis KNUE 24N045 | A. sichuanensis HKAS 107017 [26] |
|---|---|---|
| Colony | PDA, MEA, 25℃, 7 d | PDA, 25℃, 7 d |
| Color | PDA, front, pale grey to white; reverse light pink near center, and ivory edges MEA, front, pale grey; reverse yellow brown | PDA, front, dirty white; reverse yellowish brown |
| Size | PDA, 68-77 mm MEA, 68–70 mm | PDA, 55 mm |
| Shape | PDA, circular, flat, smooth, downy MEA, circular, flat, smooth, and downy | PDA, irregular, flat or effuse, medium dense, margin and fimbriate |
| Asci | Hyaline, polysporous, unitunicate, clavate to cylindric-clavate, long pedicellate, thin-walled, and apically rounded to truncate | Long, polysporous, unitunicate, clavate to cylindric-clavate, long pedicellate, thin-walled, apically rounded to truncate |
| Ascospore | Crowded, hyaline to pale yellowish or yellow-brown, long ellipsoidal to allantoid, aseptate, slightly curved, smooth-walled 7.86 (6.88–9.27) × 2.61 (2.16–3.26) μm | Crowded, initially hyaline, becoming pale yellowish to brown when mature, oblong to allantoid, aseptate, slightly curved, and smooth-walled 8.5(6–11) × 2.7(2–3) µm |
PDA: potato dextrose agar; MEA: malt extract agar.
Beltrania pseudorhombica Hua Zheng, X.Q. Yang, J.S. Deng, J.P. Xu & Z.F. Yu, Int J Syst Evol Microbiol 70 (2):1180 (2019) [MB#831904] (Fig. 4)
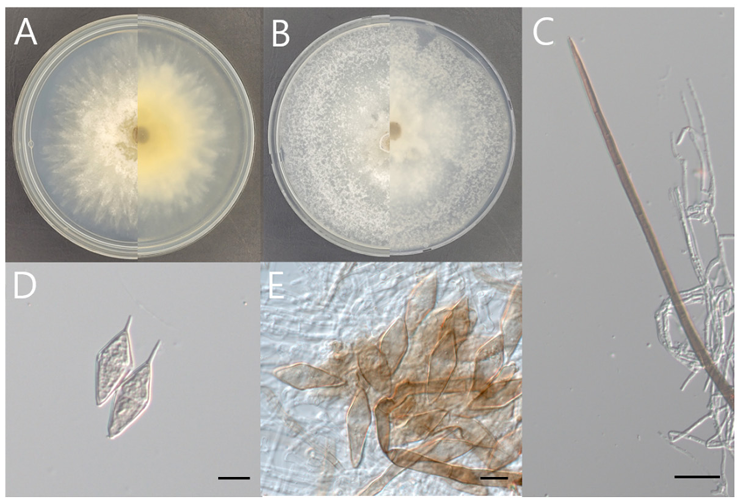
Fig. 4. Morphology of Beltrania pseudorhombica KNUE 23P958. A: Colony grown for 7 d on potato dextrose agar (PDA), B: Colony grown for 7 d on malt extract agar (MEA), C: Setae, D and E: Conidia. Scale bars: C, 20 μm; D and E, 10 μm
Morphological Characteristics: After culturing on PDA and MEA at 25℃ for 7 d, the macroscopic features were assessed. The colony diameter ranged 76–80 mm on PDA, grew circularly with radiated margins. The central part of the white mycelia exhibits a light-brown hue with cotton-like aerial mycelia. The upper mycelia develop in distinct layers, forming lobate margins resembling open flowers. The reverse side displayed colors ranging from light brown to beige and darkened towards the center. Colonies filled a 90 mm Petri dish when culture on MEA, exhibiting a soft texture with broadly spreading mycelia. The front was white to light grey, whereas the reverse ranged from light ivory to light grey. The setae were dark brown with thick walls, tapering towards the tips, and appearing straight or slightly curved with septa. The conidia were light beige-to-pale brown and biconic, with slender apical appendages protruding from the ends. A translucent, light-brown transverse band developed, and the conidia were solitary. Conidia measured 25.79 (22.36–28.9) × 9.88 (8.47–12.47) μm (n=20).
Specimen Examined: Mungyeong-si, Gyeongsangbuk-do, Korea; 36° 39′ 44.824.”N 128° 10′ 33.038.”E, September 22, 2023, B. pseudorhombica, isolated from leaf of Z. piperitum (L.) DC., strain KNUE 23P958, NIBRFGC000512626, GenBank No. PQ610921 (ITS), and PQ611014 (LSU).
Notes: The genus Beltrania was first reported in 1882 along with B. rhombica Penz. and was isolated from Citrus limonum Risso. To accommodate similar genera, the Beltraniaceae Nann. were established, including Beltrania, Beltraniella Subram., Beltraniopsis Bat. & J.L. Bezerra, Parapleurotheciopsis P.M. Kirk, and Pseudobeltrania Henn. Species in the genus Beltrania are characterized by setae without radiate branching, conidiogenous cells composed of multiple separate cells with short, tooth-like projections. The conidia are biconic with translucent transverse bands and tube-shaped ends [27,28]. B. pseudorhombica was reported in 2014, and although members of the family Beltraniaceae are not common plant pathogens, this species has been identified as a pathogen that causes leaf and fruit spots on pistachio [29]. The microscopic characteristics of strain KNUE 23P958 correspond to those of B. pseudorhombica CBS138003 [30] (Table 2).
Table 2. Morphological characteristics of strains KNUE 23P958 and CBS138003 of Beltrania pseudorhombica
| Characteristics | B. pseudorhombica KNUE 23P958 | B. pseudorhombica CBS138003 [30] |
|---|---|---|
| Colony | PDA, MEA, 25℃, 7 d | PDA, MEA, OA, 22℃, 14 d |
| Color | PDA, front, white, light brown near center; reverse, light brown to beige, and darker brown near the center MEA, front, white to light grey; reverse light ivory to light grey | PDA, front and reverse, dirty white MEA, front and reverse, dirty white OA, surface iron-grey, and outer region dirty white |
| Size | PDA, 76–80 mm diameter MEA filled a 90 mm diam petri dish | PDA, no observation MEA, 70 mm diameter OA, no observation |
| Shape | PDA, circular, flat, fluffy aerial mycelium, filamentous, and alobate margins MEA, circular, flat, smooth | PDA, no observation MEA, spreading, fluffy aerial mycelium, and lobate margin OA, no observation |
| Conidia | Solitary, biconic, pale beige to light brown, median transverse band of light brown, apical appendage, and tapering to an acutely rounded tip 25.79 (22.36–28.9) × 9.88 (8.47–12.47) μm | Solitary, biconic, pale brown, aseptate, distinct median transverse band of lighter pigment, apical appendage 7–11 × 1 µm, and tapering to an acutely rounded tip (20–)22–25(–26) × (7–)8(–9) µm |
| Setae | Dark brown, thick-walled, and gradually thinner towards the end | Erect, dark brown, thick-walled, indistinctly septate, straight to somewhat flexuous, tapering to an acute apex, and up to 5-septate |
PDA: potato dextrose agar; MEA: malt extract agar; OA, oatmeal agar.
In the present study, we successfully identified two previously unrecorded endophytic fungal species in Korea: A. sichuanensis and B. pseudorhombica. Both species, belonging to the order Xylariales, were isolated from two distinct host plants, Z. piperitum and J. rigida. Detailed morphological and molecular analyses confirmed their phylogenetic relationship and established their presence in new records in Korea. These findings expand our understanding of fungal biodiversity in Korea and highlight the significance of continued exploration and documentation of endophytic fungi to uncover their ecological roles and potential contributions to natural ecosystems.
The authors declare that there are no conflicts of interest.
This work was supported by a grant from the National Institute of Biological Resources (NIBR202402104) funded by the Ministry of Environment (MOE) of the Republic of Korea.
1. Petrini O. Fungal endophytes of tree leaves. In: Andrews JH, Hirano SS, editors. Microbial ecology of leaves. New York: Springer; 1991. p. 179-97. [DOI]
2. Khare E, Mishra J, Arora NK. Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol 2018;9:2732. [DOI]
3. Sánchez Márquez M, Bills GF, Zabalgogeazcoa I. The endophytic mycobiota of the grass Dactylis glomerata. Fungal Divers 2007;27:171-95.
4. Collado J, Platas G, González I, Peláez F. Geographical and seasonal influences on the distribution of fungal endophytes in Quercus ilex. New Phytol 1999;144:525-32. [DOI]
5. Fisher PJ, Graf F, Petrini LE, Sutton BC, Wookey PA. Fungal endophytes of Dryas octopetala from a high arctic polar semidesert and from the Swiss Alps. Mycologia 1995;87:319-23. [DOI]
6. Arnold AE, Lutzoni F. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 2007;88:541-9. [DOI]
7. Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA. Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci U S A 2003;100:15649-54. [DOI]
8. Zabalgogeazcoa I. Fungal endophytes and their interaction with plant pathogens: a review. Span J Agr Res 2008;6:138-46. [DOI]
9. Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 2003;67:491-502. [DOI]
10. Smith GJ, Liew EC, Hyde KD. The Xylariales: a monophyletic order containing 7 families. Fungal Divers 2003;13:185-218.
11. Barr ME. Prodromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon 1990;39:43-184.
12. Hawksworth DL, Kirk P, Sutton B, Pegler D. Ainsworth & Bisby’s dictionary of the fungi. 10th ed. Oxon: CABI Publishing; 1996. [DOI]
13. Rogers JD. The Xylariaceae: systematic, biological and evolutionary aspects. Mycologia 1979;71:1-42. [DOI]
14. Whalley A. The xylariaceous way of life. Mycol Res 1996;100:897-922. [DOI]
15. Becker K, Stadler M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J Antibiot 2021;74:1-23. [DOI]
16. Eo JK, Choi JW, Eom AH. Diversity, distribution, and host plant of endophytic fungi: a focus on Korea. Mycobiology 2022;50:399-407. [DOI]
17. Cha JE, Kim YJ, Kim JW, Eom AH. Two new records of endophytic fungi isolated from Lindera obtusiloba in Korea: Colletotrichum citricola and Valsa ceratophora. Kor J Mycol 2024;52:135-43.
18. Dos Santos GD, Gomes RR, Gonçalves R, Fornari G, Maia BHLNS, Schmidt-Dannert C, Gaascht F, Glienke C, Schneider GX, Colombo IR, et al. Molecular identification and antimicrobial activity of foliar endophytic fungi on the brazilian pepper tree (Schinus terebinthifolius) reveal new species of Diaporthe. Curr Microbiol 2021;78:3218-29. [DOI]
19. Harris JL. Modified method for fungal slide culture. J Clinical Microbiol 1986;24:460-1. [DOI]
20. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8. [DOI]
21. Kruys Å, Eriksson OE, Wedin M. Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycol Res 2006;110:527-36. [DOI]
22. Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 1995;61:1323-30. [DOI]
23. Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 2021;38:3022-7. [DOI]
24. Maharachchikumbura SSN, Wanasinghe DN, Elgorban AM, Al-Rejaie SS, Kazerooni EA, Cheewangkoon R. Brunneosporopsis yunnanensis gen. et sp. nov. and Allocryptovalsa xishuangbanica sp. nov., new terrestrial Sordariomycetes from Southwest China. Life 2022;12:635. [DOI]
25. Zhu H, Pan M, Wijayawardene NN, Jiang N, Ma R, Dai D, Tian C, Fan X. The hidden diversity of diatrypaceous fungi in China. Front Microbiol 2021;12:646262. [DOI]
26. Samarakoon MC, Hyde KD, Maharachchikumbura SSN, Stadler M, Gareth Jones EB, Promputtha I, Suwannarach N, Camporesi E, Bulgakov TS, Liu JK. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers 2022;112:1-88. [DOI]
27. Zheng H, Yang XQ, Deng JS, Xu JP, Yu ZF. Beltrania sinensis sp. nov., an endophytic fungus from China and a key to species of the genus. Int J Syst Evol Microbiol 2020;70:1178-85. [DOI]
28. Seifert KA, Gams W. The genera of Hyphomycetes-2011 update. Persoonia 2011;27:119-29. [DOI]
29. Lichtemberg P, Moral J, Sherman J, Nouri MT, Lake J, Felts DG, Michailides TJ. Characterizing Beltrania pseudorhombica the causal agent of pistachio leaf and fruit spot in Arizona. Eur J Plant Pathol 2019;154:849-54. [DOI]
30. Crous PW, Shivas RG, Quaedvlieg W, Bank Mvd, Zhang Y, Summerell BA, Guarro J, Wingfield MJ, Wood AR, Alfenas AC, et al. Fungal Planet description sheets: 214-280. Persoonia 2014;32:184-306. [DOI]