Jun-Woo Choi1, Seo-Ryeong Lee1, Gwang-Jae Lim1, Jin-Sil Choi1, Chang-Gi Back2, Seung-Yeol Lee1,3*, and Hee-Young Jung1,3
1Department of Plant Medicine, Kyungpook National University, Daegu 41566, Korea
2Department of Environmental Horticulture and Landscape Architecture, Environmental Horticulture, Dankook University, Cheonan 31116, Korea
3Institute of Plant Medicine, Kyungpook National University, Daegu 41566, Korea
*Correspondence to leesy1123@knu.ac.kr
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 371-380.
https://doi.org/10.4489/kjm.520415
Received on November 04, 2024, Revised on December 16, 2024, Accepted on December 16, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
In August 2023, apple fruits exhibiting flyspeck signs were collected from orchards in Cheongsong-gun and Muju-gun, located in the Gyeongbuk and Jeonbuk provinces, during an investigation of apple diseases. Eleven fungal strains were isolated from the flyspeck signs on these apple fruits. To observe the cultural and morphological characteristics, the isolated fungal strains were cultured on potato dextrose agar, malt extract agar, and oatmeal agar at 25℃ in the dark for 2 weeks. Subsequently, conidia, conidiophores, and conidiogenous cells of the strains were examined. Internal transcribed spacer regions, translation elongation factor 1-α, and β-tubulin genes of the isolated strains were amplified for phylogenetic analysis. Based on cultural and morphological characteristics, alongside phylogenetic analysis, the strains were identified as Schizothyrium emperorae, S. pomi, and S. wisconsinense, respectively. The pathogenicity test confirmed that flyspeck symptoms were exhibited on inoculated apple fruits. In a previous study, Peltaster fructicola was reported to be the causal agent of sooty blotch and flyspeck disease in apples in Korea. To our knowledge, this is the first report of flyspeck disease caused by S. emperorae on apple fruits, alongside the identification of a new host for S. wisconsinense in Korea.
Apple, Flyspeck, Schizothyrium emperorae, Schizothyrium wisconsinense
Apples (Malus pumila Mill., M. domestica Borkh.) are significant cash crops globally and represent one of the most valuable fruits in Korea. In 2023, apple cultivation spanned 394,428 ha [1]. However, these fruits exhibit high susceptibility to fungal pathogens, which significantly affects both yield and fruit quality, leading to substantial economic losses [2]. Among these pathogens, sooty blotch and flyspeck (SBFS) manifest as blemishes and dark smudges on the fruit surface, typically occurring during the late season in humid regions [3]. The SBFS fungi colonize the epicuticular wax layer of various fruits, such as apples, pears, bananas, and persimmons, without penetrating the flesh [4]. While these fungi do not cause physiological damage, their presence on the fruit surface significantly reduces the market value of the affected produce [5]. The SBFS diseases are caused by at least 30 genera, including Schizothyrium, Zygophiala, Peltaster, and Dissoconium, belonging to the phyla Ascomycota and Basidiomycota [6]. The morphology of SBFS signs, known as mycelial type, varies depending on the genus or species involved. Mycelial types are categorized based on the presence or absence of sclerotium-like bodies and mycelial mats. When sclerotium-like bodies are present without mycelial mats, this is classified as the “speck type”, which can be further divided into three subtypes based on the size and density of the sclerotium-like bodies: flyspeck, compact speck, and discrete speck [7]. The primary genera causing flyspeck signs on plants are Schizothyrium and Zygophiala. The genus Schizothyrium, established by Desmazières in 1849, includes a range of epiphytic fungi, including flyspeck pathogens such as S. pomi, S. wisconsinense, and S. cryptogamum [8]. In Korea, S. pomi, S. wisconsinense, and S. jamaicense have been identified as causal agents of flyspeck on various fruits [9]. In August 2023, apple fruits exhibiting signs of flyspeck were collected from apple orchards in Cheongsong-gun and Muju-gun. Subsequently, the fungal species were isolated and identified using cultural and morphological characteristics, along with phylogenetic analysis. This study is the first report of previously undescribed species causing flyspeck on apple fruits in Korea.
A disease survey was conducted in apple orchards across the Gyeongbuk and Jeonbuk provinces in August 2023. Apple fruits (cv. Fuji) and crab apples (Malus prunifolia Borkh.) exhibiting flyspeck signs were collected from orchards in Cheongsong-gun (36°17’09.8″N 128°57’31.2″E and 36°16′13.2″N 128° 52′52.2″E) and Muju-gun (35°57’03.5″N 127°51’58.3″E). Medjedović et al. reported the isolation of SBFS species from affected fruit surfaces [10]. Apple fruits were disinfected by wiping with 70% ethanol. Subsequently, flyspeck colonies were transferred to potato dextrose agar (PDA; Difco, Detroit, MI, USA) and incubated at 25℃ in the dark. After two weeks of cultivation, small colonies were observed, and the margins of each colony were subcultured onto fresh PDA media. The isolated strains KNUF-23-CS01 and KNUF-23-MJ01 were deposited at the Korean Collection for Type Cultures (KCTC).
The mycelial types present on the apple surfaces were examined. The cultural characteristics of the fungal strains were assessed using PDA, malt extract agar (MEA; Difco, Detroit, MI, USA), and oatmeal agar (OA; Difco, Detroit, MI, USA), following previous study [11]. Agar plugs were transferred from the edges of the colonies on PDA to each medium using a 4 mm cork borer and incubated in the dark at 25℃ for 2 weeks. The colony size was measured using vernier calipers (Mitutoyo, Japan). Overall, 30 measurements were taken to observe the conidia alongside the fungal structures, including conidiophores, apical cells, and conidiogenous cells.
The genomic DNA of the isolated strains was extracted using the HiGene™ Genomic DNA Prep Kit (BIOFACT, Daejeon, Korea), following the instructions of the manufacturer. To amplify the internal transcribed spacer (ITS) regions, translation elongation factor 1-α (TEF), and β-tubulin (TUB2) genes, primer pairs ITS1F/ITS4, EF1-728F/EF1-986R, and Bt2a/Bt2b were used for all isolated strains, respectively [12–15]. The sequences of other Schizothyrium species were retrieved from the National Center for Biotechnology Information (NCBI) GenBank for phylogenetic analysis (Table 1). Alignments were performed using the Clustal X program. After which ambiguous regions were removed. The TamuraNei model was used to calculate evolutionary distance matrices for the maximum likelihood (ML) method [16]. Phylogenetic trees were constructed using the ML method with Molecular Evolutionary Genetics Analysis (MEGA) 11.0 software, incorporating 1,000 bootstrap replications [17]. The obtained sequences of ITS regions, TEF, and TUB2 were registered in the NCBI (Table 1).
Table 1. GenBank accession numbers for strains used in phylogenetic analysis
| Species name | Strain number | GenBank accession numbers | ||
|---|---|---|---|---|
| ITS | TEF | TUB2 | ||
| Schizothyrium cryptogamum | HL-MMQSG-1 | KF806029 | KJ176948 | KJ176977 |
| Schizothyrium cylindricum | ZMHS-8 | KF956056 | KJ176957 | KJ176983 |
| Schizothyrium cylindricum | ZMHS-53 | KF956059 | KJ176956 | KJ176984 |
| Schizothyrium emperorae | CC-WWZ-2 | KF806031 | KJ176962 | KJ176974 |
| Schizothyrium emperorae | GL-QXLPPG-1T | KF646710 | KJ176941 | KJ176970 |
| Schizothyrium emperorae | GL-QXLPPG-2 | KF806025 | KJ176942 | KJ176971 |
| Schizothyrium emperorae | LYP-YX-12-1 | KF806022 | KJ176952 | KJ176981 |
| Schizothyrium emperorae | KNUF-23-CS01 | PQ416027 | PQ424600 | PQ424592 |
| Schizothyrium inaequale | GL-MMXJ-56A | KF806032 | KJ176943 | KJ176972 |
| Schizothyrium inaequale | GL-MMXJ-52T | KF646709 | KJ176945 | KJ578753 |
| Schizothyrium musae | CHC-HNBJ-2T | KF646707 | KJ176946 | KJ176975 |
| Schizothyrium pomi | CUA1aT | EF164898 | KJ176961 | KJ578755 |
| Schizothyrium pomi | PEA1a | KJ578752 | KJ730239 | KJ578754 |
| Schizothyrium pomi | KNUF-23-CS13 | PQ416028 | PQ424601 | PQ424593 |
| Schizothyrium qianense | HL-MMGL-2 | KF806028 | KJ176947 | KJ176976 |
| Schizothyrium qianense | LWH-LNLZ-14 | KF806030 | KJ176944 | KJ176973 |
| Schizothyrium qianense | MYNXY4-8 | KF806011 | KJ176954 | KJ176982 |
| Schizothyrium qianense | MYQ-QXBS-06T | FJ769236 | KJ176955 | KJ176985 |
| Schizothyrium tardicrescens | MWA1aT | AY598856 | KJ176960 | KJ578756 |
| Schizothyrium wisconsinense | CC-SZSZ-20 | KF806023 | KJ176939 | KJ176968 |
| Schizothyrium wisconsinense | CC-ZJJ-69 | KF857347 | KJ176938 | KJ176967 |
| Schizothyrium wisconsinense | HL-CSPGL-53 | KF806026 | KJ176949 | KJ176978 |
| Schizothyrium wisconsinense | LB-15 | EU825775 | KJ176937 | KJ176966 |
| Schizothyrium wisconsinense | LHY-LB-6 | KF805997 | KJ176935 | KJ176964 |
| Schizothyrium wisconsinense | LHY-LB-12 | KF805998 | KJ176936 | KJ176965 |
| Schizothyrium wisconsinense | LHY-LB-14 | KF806020 | KJ176951 | KJ176980 |
| Schizothyrium wisconsinense | KNUF-23-CA01 | PQ416022 | PQ424599 | PQ424595 |
| Schizothyrium wisconsinense | KNUF-23-CS04 | PQ416018 | PQ424597 | PQ424594 |
| Schizothyrium wisconsinense | KNUF-23-MJ01 | PQ416026 | PQ424598 | PQ424596 |
| Zygophiala trispora | HL-HKBJ-23DT | KF646711 | KJ176950 | KJ176979 |
| Paramycosphaerella marksii | CBS:110920 | GU269644 | KF903145 | LC121230 |
ITS: internal transcribed spacer regions; TEF: translation elongation factor 1-α; TUB2: β-tubulin.
T Type strain. The strain used in this research is highlighted in bold.
To fulfill Koch’s postulates, isolated strains were inoculated onto apple fruits (Malus domestica cv. Fuji) to evaluate their pathogenicity. The pathogenicity tests were conducted using Johnson’s method with modifications [18]. Healthy apple fruits were disinfected with tap water and treated with 70% ethanol. To prepare the inoculums, 0.05 g of two-week-old colonies on PDA were homogenized with 1 mL of doubledistilled water, and Tween 20 was added to achieve a final concentration of 0.1%. Sterilized paper discs were immersed in the suspension for 2 min before being attached to healthy apple fruits. Subsequently, the inoculated apple fruits were placed in a moist chamber and incubated at 25°C for 3 weeks.
The mycelial type observed on the surface of apple fruits was identified as flyspeck, characterized by the presence of black, shiny, round, or ovoid sclerotium-like bodies without accompanying mycelial mats. These bodies measured approximately 188 µm (range: 120–282 µm) in diameter and 217 µm (range: 150–402 µm) in length (Fig. 1A and B). The two-week-old colonies cultured on PDA exhibited a flat, circular morphology with white aerial mycelium, a smooth and regular margin, and an olivaceous gray to dark green center, cream edge, measuring 9.2–12.7 mm in diameter (Fig. 1C). In contrast, the colonies on MEA presented a flat, circular form with white aerial mycelium, a smooth and regular margin, olive to dark green center, and a dirty white edge, measuring 10.8–13.7 mm in diameter (Fig. 1D). On OA, the colonies exhibited a flat, circular morphology characterized by white aerial mycelium and a smooth, regular margin. The centers of the colonies ranged from dark gray to pale gray, and pale white to cream edge, with diameters measuring 7.3–17.0 mm in diameter (Fig. 1E). The conidiophores were erect, scattered, 3-septate, and subcylindrical, displaying an irregularly flexuous structure composed of a supporting cell, a stipe, and an apical cell. The supporting cell was hyaline to subhyaline, while the stipe was smooth and dark brown, measuring 15–22 × 3–6 µm (from the basal septum to below the phialide). The apical cell at the tip of the stipe was medium brown and finely verruculose, measuring 2–4 × 4–6 µm, giving rise to one or two polyblastic conidiogenous cells that were medium brown, finely verruculose, doliiform to ellipsoidal, and featured 1–2 prominent scars. The scars were apical and lateral, darkened, thickened, somewhat refractive, and measured 2 µm in width. Conidia were solitary, fusiform to obclavate, hyaline, smooth, thick-walled, and granular, with 0–2 transverse septate. They typically measured 9–17 × 5–8 µm, though rare 2-septate forms measured 39.4 × 7.9 µm, featuring an obtuse apex, a subtruncate base, and a darkened, thickened hilum measuring 2 µm in width (Fig. 1F–J). The morphological and cultural characteristics of KNUF-23CS01 closely resembled those of the previously identified S. emperorae (Table 2).
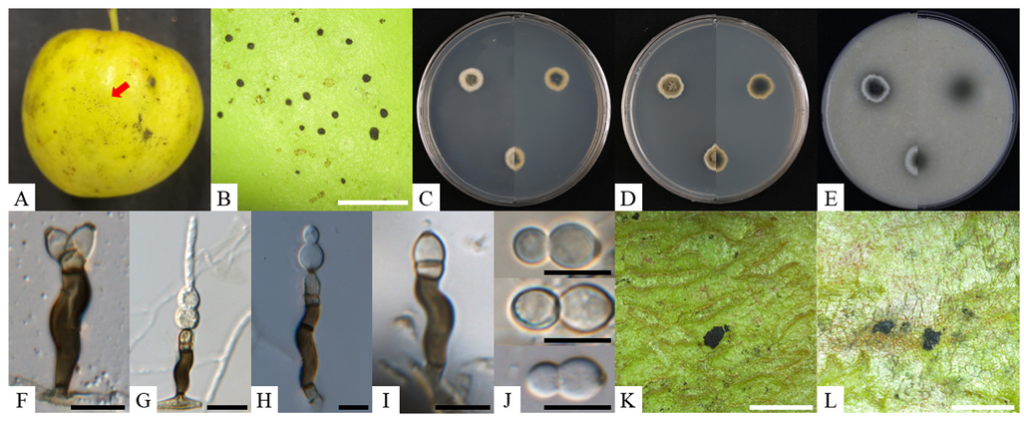
Fig. 1. Cultural and morphological characteristics of Schizothyrium emperorae KNUF-23-CS01. A, B: flyspeck signs observed on apple fruits; C–E: two-week-old colonies grown on PDA, MEA, and OA, respectively; F–J: morphological features of conidiophores and conidia; K, L: results from the pathogenicity test. Black scale bars = 10 µm, white scale bars = 2 mm. PDA: potato dextrose agar; MEA: malt extract agar; OA: oatmeal agar.
Table 2. Cultural and morphological characteristics of isolated strains with reference to Schizothyrium species
| Characteristics | S. emperorae KNUF-23 -CS01a | S. emperorae GL-QXLP PG-1Tb | S. wisconsinense KNUF-23 -MJ01a | S. wisconsinense CBS 118950Tc | |
|---|---|---|---|---|---|
| Colony | PDA | Flat, circular, smooth margins, dark gray to pale gray in the center, pale white to cream edge, 7.3–17.0 mm diam. | N/A | Flat, circular, smooth, margin, dirty white in the center, cream edge, 13.3–17.7 mm diam. | N/A |
| MEA | Flat, circular, smooth margins, olive to dark green in the middle, dirty white edge, 10.8–13.7 mm diam. | N/A | Circular, fewer aerial mycelium, smooth margin, wrinkled, bright gray in the center, flat, yellow cream edge, 13.6–16.8 mm diam. | 13.5–22.5 mm diam. | |
| OA | Flat, circular, smooth margins, dark gray to pale gray in the center, pale white to cream edge, 7.3–17.0 mm diam. | Smooth, regular margins, pale white, 25 mm diam. | Flat, circular, smooth margins, pale olivaceous gray in the center, dirty white to cream edge, 14.2–18.0 mm diam. | Flat, moderate aerial mycelium, smooth margins, pale olivaceous gray in the middle, dirty white to cream outer zone. | |
| Conidiophores | Stipes | Flexuous, smooth, dark brown, 16–26 × 4–6 µm. | Flexuous, smooth, dark brown, 16–27 × 4–6 µm. | Flexuous, smooth, dark brown 15–22 × 3–6 µm. | Flexuous, smooth, dark brown, 15–20 × 4–7 µm. |
| Apical cells | Finely verruculose, medium brown, 2–4 × 4–6 µm. | Finely verruculose, medium brown, 3–6 × 4–6 µm. | Finely verruculose, medium brown, 2–4 × 3–5 µm. | Finely verruculose, medium brown, 3–4 × 4–5 µm. | |
| Conidiogenous cells | Finely verruculose, medium brown, doliiform to ellipsoidal, polyblastic, 4–8 × 4–6 µm, with 1–2 scars. | Finely verruculose, medium brown, doliiform to ellipsoidal, polyblastic, 4–8 × 4–6 µm, with 1–2 scars. | Finely verruculose, medium brown, doliiform to ellipsoidal, polyblastic, 6–8 × 4–6 µm, with 1–2 scars. | Finely verruculose, medium brown, doliiform to ellipsoidal, polyblastic, 7–11 × 5–6 µm, with 1–2 scars. | |
| Conidia | Solitary, fusiform to obclavate, hyaline, transversely 0–2 septate, 1-septate, 12–17 × 5–8 µm, or rarely 2-septate, 39.4 × 7.9 µm. | Solitary, fusiform to obclavate, hyaline, transversely 0–2 septate, 1-septate, 15–20 × 6–8 µm, or 2-septate, 18–28 × 6–9 µm. | Solitary, fusiform to obclavate, hyaline, aseptate, 6–9 × 5–7 µm, or transversely 1-septate, 11–17 × 6–8 µm. | Solitary, fusiform to obclavate, hyaline, aseptate 6–8 × 6–8 µm or transversely 1-septate 15–18 × 7–8 µm. | |
| Mycelial type | Flyspeck, 188 (120–282) × 217 (150–402) µm. | Flyspeck. | Flyspeck, 362 (262–508) × 423 (358–566) µm. | Flyspeck, 380 (300–450) × 500 (425–600) µm. |
a Fungal strain investigated in this study; b Source of description [20]; c Source of description [11]; T Type strain.
PDA: potato dextrose agar; MEA: malt extract agar; OA: oatmeal agar; N/A: not available in previous study.
Partial sequences of the ITS regions (559 bp), TEF (363 bp), and TUB2 (397 bp) were obtained from the isolated strains. Basic Local Alignment Search Tool (BLAST) analysis revealed that the ITS regions sequence exhibited 99.5% similarity with S. emperorae GL-QXLPPG-1T and 98.4–99.2% similarity with several S. wisconsinense strains. The partial sequence of the TEF exhibited 98.1% similarity with S. emperorae strain GL-QXLPPG-1T, and below 90% similarity with S. wisconsinense strains. For the TUB2 gene, the obtained sequence exhibited 99.7% similarity with S. emperorae strain GL-QXLPPG-1T, and 94.7–95.2% similarity with S. wisconsinense strains. ML phylogenetic trees were constructed using the sequences of the ITS regions, TEF, and TUB2. The KNUF-23-CS01 was clustered with S. emperorae strains, forming a distinct cluster from S. wisconsinense strains (Fig. 3).
The nine isolated strains exhibited similar cultural and morphological characteristics. Their sequences of the ITS regions, TEF and TUB2, were identical, resulting in their clustering within phylogenetic trees. Consequently, the KNUF-23-MJ01 strain was selected as the representative strain for further analysis of cultural and morphological characteristics.
The mycelial type observed on the surface of apple fruits exhibited flyspeck characteristics, lacking a mycelial mat, and displayed black, shiny, round or ovoid, sclerotium-like bodies, measuring 362 (262–508) × 423 (358–566) µm in diameter (Fig. 2A and B). The two-week-old colonies grown on PDA were flat and circular, displaying white aerial mycelium with a smooth, regular margin. The centers appeared dirty white, bordered by a large, cream edge, reaching 13.3–17.7 mm in diameter (Fig. 2C). On MEA, colonies were circular with fewer white aerial mycelium and smooth, regular margins. The centers appeared bright gray and were surrounded by a wrinkled surface with a large, flat, yellow-cream edge, measuring 13.6–16.8 mm in diameter (Fig. 2D). On OA, colonies exhibited flat, circular morphology with white aerial mycelium and smooth, regular margins. The centers were pale olivaceous gray, surrounded by a large, dirty white to cream edge, reaching 14.2–18.0 mm in diameter (Fig. 2E). The conidiophore was erect and scattered, composed of 3-septate and subcylindrical, irregularly flexuous structure, consisting of a supporting cell, stipe, and apical cell. The stipe, originating from a hyaline or subhyaline supporting cell, was smooth and dark brown, measuring 13–23 × 4–6 µm (from the basal septum to below the phialide). The apical cell at the tip of the stipe was a finely verruculose, medium brown structure measuring 2–4 × 4–5 µm. It supported one or two conidiogenous cells, which were medium brown, finely verruculose, doliiform to ellipsoidal, polyblastic. Each conidiogenous cell had 1–2 prominent apical and lateral scars that were darkened, thickened, and somewhat refractive, measuring 2 µm in width. Conidia were solitary, fusiform to obclavate, hyaline, smooth, and thick-walled with a granular. Aseptate conidia measured 6–9 × 5–7 µm, or transversely 1-septate, 11–17 × 6–8 µm, an obtuse apex, a subtruncate base, and a darkened, thickened hilum measuring 2 µm wide (Fig. 2F–J). The morphological and cultural characteristics of the nine strains were consistent with those of previously identified S. wisconsinense (Table 2).
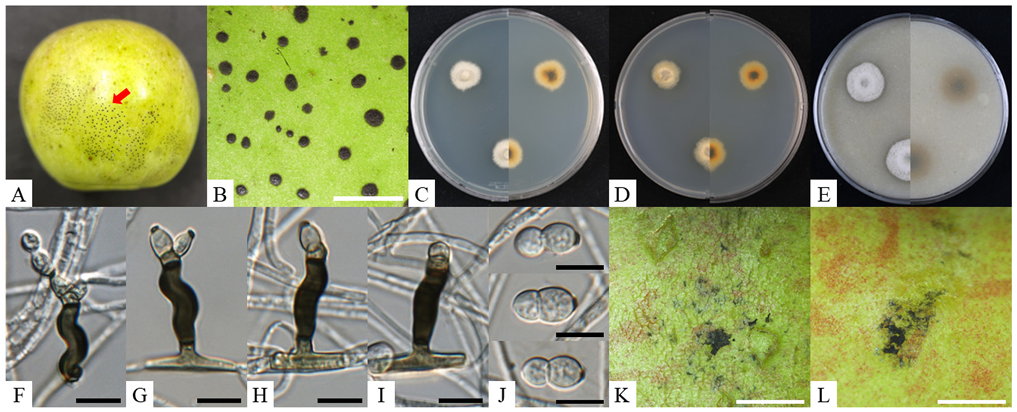
Fig. 2. Cultural and morphological characteristics of Schizothyrium wisconsinense KNUF-23-MJ01. A, B: flyspeck signs observed on apple fruits; C–E: two-week-old colonies grown on PDA, MEA, and OA, respectively; F–J: morphological features of conidiophores and conidia; K, L: result from the pathogenicity test. Black scale bars = 10 µm, white scale bars = 2 mm. PDA: potato dextrose agar; MEA: malt extract agar; OA: oatmeal agar.
Amplification of the ITS regions, TEF, and TUB2 from the isolated strains produced fragments of 564, 232, and 397 bp, respectively. The ITS regions sequences revealed 99.6–99.8% similarity with S. wisconsinense strains (CC-ZJJ-69 and LHY-LB-15), and 98.7% similarity with S. emperorae strains. The partial TEF and TUB2 sequences showed more than 99.5% similarity with S. wisconsinense strains. In contrast the similarity with S. emperorae strains for TEF and TUB2 were below 90.6% and 95.2%, respectively. An ML phylogenetic tree was generated using concatenated sequences of ITS regions, TEF, and TUB2, showing that the nine isolated strains clustered with S. wisconsinense strains (Fig. 3).
After three weeks, dark, circular, or irregular flyspeck appeared on apples inoculated with KNUF-23CS01 and KNUF-23-MJ01. The signs were similar to those observed on the fruits from which the two strains were originally isolated (Fig. 1K and L; Fig. 2K and L). Additionally, the cultural characteristics of fungi re-isolated from the inoculated apples matched those of KNUF-23-CS01 and KNUF-23-MJ01.
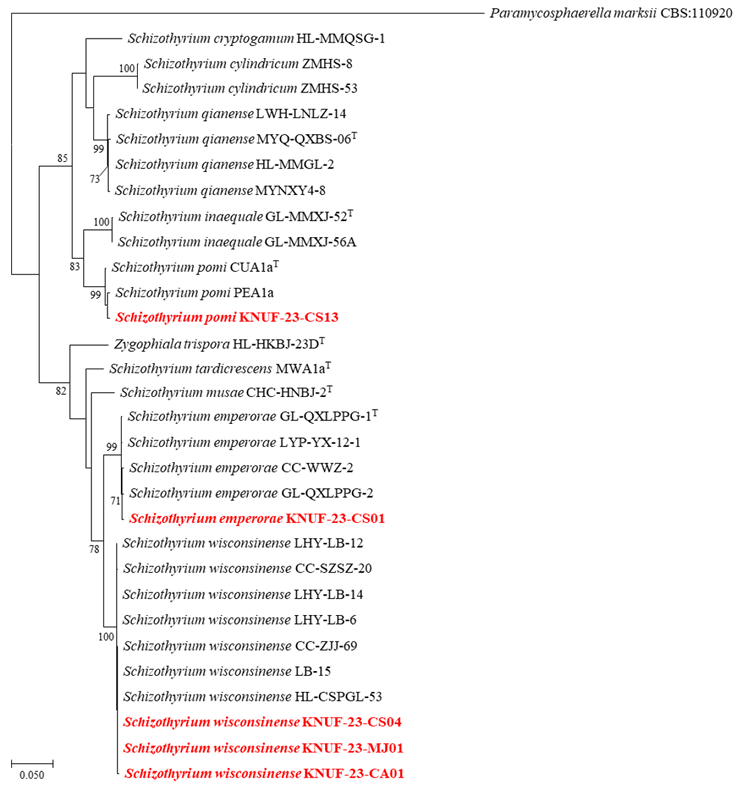
Fig. 3. Maximum likelihood (ML) phylogenetic tree of isolated strains based on the sequences of internal transcribed spacer (ITS) regions, translation elongation factor 1-α (TEF), and β-tubulin (TUB2) genes. The tree illustrates relationships between isolated strains and closely related Schizothyrium species. Paramycosphaerella marksii (CBS: 110920) was used as an outgroup. Bootstrap values (> 70%) obtained from 1,000 replicates are indicated above the branches. The strain isolated in this study is highlighted in red and bold. Scale bar = 0.050 substitutions per nucleotide position.
The flyspeck pathogen was first reported in 1834 as Labrella pomi (= S. pomi) on pear [19]. In 1945, E.W. Mason established the genus Zygophiala, with the type species Z. jamaicensis causing flyspeck on banana [19]. To date, 11 Zygophiala species have been described globally, associated with flyspeck signs on various plants [20]. In 2015 and 2016, several species of Zygophiala were reclassified into the genus Schizothyrium, including S. wisconsinense (= Z. wisconsinensis), S. cryptogamum (= Z. cryptogama), and S. emperorae (= Z. emperorae) [21,22]. In Korea, several Schizothyrium species have been reported as causal agents of flyspeck disease on sweet persimmon, apple, grape, and Chinese flowering quince, specifically S. wisconsinense, S. pomi, and S. jamaicense. Among these species, S. wisconsinense has been reported as a pathogen agent of sweet persimmon in Korea and has been documented on apple fruits in various regions, such as the United States, China, and Europe [6,23]. However, S. wisconsinense has not been identified as a causal agent of apple fruit disease in Korea [9]. In 2014, S. emperorae was first reported as the causal agent of flyspeck on apple fruits in China. However, it has not been documented in other countries [20]. The nine strains and KNUF-23-CS01 were identified as S. wisconsinense and S. emperorae, respectively, based on cultural and morphological characteristics, alongside phylogenetic analysis. Consequently, this study represents the first report of the previously unrecorded species S. emperorae and a new host for S. wisconsinense, causing flyspeck symptoms on apple fruits in Korea.
The authors declare that they have no potential conflicts of interest.
This research was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development” (Project No. RS-2024-00396930), Rural Development Administration, Republic of Korea.
1. KOSIS (Korea Statistical Information Service). Fruits production [Internet]. Daejeon: Statistics Korea; 2023 [cited 2023 Dec 22]. Available from: https://kosis.kr.
2. Patriarca A. Fungi and mycotoxin problems in the apple industry. Curr Opin Food Sci 2019;29:42-7. [DOI]
3. Batzer JC, Prado MM, Svendsen JM, Gleason ML. Diversity of sooty blotch and flyspeck fungi on cider apples in Spain. PhytoFront 2022;2:289-306. [DOI]
4. Gleason ML, Batzer JC, Sun G, Zhang R, Arias MMD, Sutton TB, Crous PW, Ivanović M, McManus PS, Cooley DR, et al. A new view of sooty blotch and flyspeck. Plant Dis 2011;95:368-83. [DOI]
5. Zhang M, Gao L, Shang S, Han X, Zhang R, Latinović J, Latinović N, Batzer JC, Gleason ML, Sun G. New species and record of Zygophiala (Capnodiales, Mycosphaerellaceae) on apple from Montenegro. Phytotaxa 2015;195:227-35. [DOI]
6. Gleason ML, Zhang R, Batzer JC, Sun G. Stealth pathogens: The sooty blotch and flyspeck fungal complex. Annu Rev Phytopathol 2019;57:135-64. [DOI]
7. Batzer JC, Gleason ML, Harrington TC, Tiffany LH. Expansion of the sooty blotch and flyspeck complex on apples based on analysis of ribosomal DNA gene sequences and morphology. Mycologia 2005;97:1268-86. [DOI]
8. Phookamsak R, Boonmee S, Norphanphoun C, Wanasinghe DN, de Silva NI, Dayarathne MC, Hongsanan S, Bhat DJ, Hyde KD. Schizothyriaceae. Mycosphere 2016;7:154-89. [DOI]
9. Yoon SH, Hong SB, Choi YJ, Lee DH, Lee SY, Choi HY, Lee SH, Choi IS, Kim DG, Kim YH, et al. List of plant diseases in Korea 6th edition. Seoul: The Korean Society of Plant Pathology; 2023. p. 420-1.
10. Medjedović A, Frank J, Schroers HJ, Oertel B, Batzer JC. Peltaster cerophilus is a new species of the apple sooty blotch complex from Europe. Mycologia 2014;106:525-36. [DOI]
11. Batzer JC, Arias MMD, Harrington TC, Gleason ML, Groenewald JZ, Crous PW. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 2008;100:246-58. [DOI]
12. Gardes M, Bruns TD. ITS primers with enhanced specificity for Basidiomycetes – application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8. [DOI]
13. White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: A guide to methods and applications. In: Innis MA, Gelfand DH, Sninsky JJ, editors. San Diego: Academic Press; 1990. p. 315-22. [DOI]
14. Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia 1999;91:553-6. [DOI]
15. Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl Environ Microbiol 1995;61:1323-30. [DOI]
16. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 1993;10:512-26.
17. Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 2021;38:3022-7. [DOI]
18. Johnson EM, Sutton TB, Hodges CS. Etiology of apple sooty blotch disease in North Carolina. Phytopathology 1997;87:88-95. [DOI]
19. Williamson SM, Sutton TB. Sooty blotch and flyspeck of apple: etiology, biology, and control. Plant Dis 2000;84:714-24. [DOI]
20. Gao L, Zhang M, Zhao W, Hao L, Chen H, Zhang R, Batzer JC, Gleason ML, Sun G. Molecular and morphological analysis reveals five new species of Zygophiala associated with f lyspeck signs on plant hosts from China. PLoS ONE 2014;9:e110717. [DOI]
21. Rossman AY, Crous PW, Hyde KD, Hawksworth DL, Aptroot A, Bezerra JL, Bhat JD, Boehm E, Braun U, Boonmee S, et al. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus 2015;6:507-23. [DOI]
22. Rossman AY, Allen WC, Braun U, Castlebury LA, Chaverri P, Crous PW, Hawksworth DL, Hyde KD, Johnston P, Lombard L, et al. Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus 2016;7:289-308. [DOI]
23. Kim J, Choi O, Kwon JH. First report of flyspeck caused by Zygophiala wisconsinensis on sweet persimmon fruit in Korea. Plant Dis 2011;95:616. [DOI]