Ye-Yeong Kim, Mi-Jeong Park, Yeongseon Jang*, and Kang-Hyeon Ka
Forest Microbiology and Application Division, Forest Bioresources Department, National Institute of Forest Science, Suwon 16631, Korea
*Correspondence to idjys@korea.kr
Korean Journal of Mycology (Kor J Mycol) 2025 March, Volume 53, Issue 1, pages 37-46.
https://doi.org/10.4489/kjm.2025.53.1.5
Received on February 28, 2025, Revised on March 20, 2025, Accepted on March 20, 2025, Published on Mar 31, 2025.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Urban forests play a crucial role in environmental improvement, recreation, and education. However, their development has led to decreased biodiversity and forest simplification. Reduced biodiversity increases vulnerability to pathogens, especially heart-rot fungi, which decompose tree tissues, weaken, and ultimately topple trees. In this study, we investigated the diversity of wood-decay fungi, including heart-rot fungi, in an urban Hongneung Forest. A total of 94 samples were collected and identified through morphological and molecular analyses of the internal transcribed spacer (ITS) region of the DNA sequences. A total of 32 species, 26 genera, 18 families, and 11 orders were identified. Most species belonged to Polyporales, accounting for 59% (19 species) of all species. At the genus level, Perenniporia, Neohypochnicium, Coniophora, Ceratobasidium, and Scytinostroma were the most prevalent. Heart-rot fungi, which decay living trees, constituted 13% of all species observed, including Bjerkandera adusta, Coniophora arida, Perenniporia fraxinea, and Somion delectans. These fungi were primarily distributed in the lower parts or trunks of trees. Nucleotide-level ITS sequence analysis identified genetic differences between C. arida (four specimens) and P. fraxinea (five specimens). Two C. arida and four P. fraxinea variants were found in the forests. This study provides baseline data on the diversity of wood-decay fungi in Hongneung Forest, which can be used for future research on heart-rot fungi in urban Korean forests.
Diversity, Heart-rot fungi, Internal transcribed spacer, Urban forests
Recently, urban forests have become increasingly important for enhancing urban living environments and providing spaces for relaxation and environmental education [1]. However, in the process of restoring urban forests damaged by urban development, the focus has often been on artificial afforestation and erosion control rather than on restoring the original ecosystems [2]. As a result, urban forest ecosystem risk becomes unstable, with a decline in biodiversity and growing concern over the simplification of forest structures [2].
As species diversity decreases, forests become more vulnerable to pathogen attack [3]. Pathogens damage millions of trees in forests every year [4]. Certain wood decaying fungi can invade and kill the sapwood of living trees, ultimately leading to their death [5]. These fungi inhabit the heartwood of their living hosts and are commonly referred to as heart-rot fungi [6]. Heart-rot breaks down the hemicellulose, cellulose, and lignin in trees, leading to their collapse [7]. In urban environments, falling trees or branches can cause injury and property damage [8].
Seoul is the capital of South Korea. It has an area of 605 km2, and approximately 26.7% is covered by green spaces [9]; however, it has been fragmented due to land development [10]. Hongneung Forest is important as a place for rest and relaxation and for preserving the habitat and species diversity of wild animals [11]. The forest contains 20,000 plants from 157 families and 2,035 species, including 1,224 woody (836 native and 388 foreign) and 811 herbaceous species [12]. Urban forests such as Hongneung Forest in South Korea provide unique habitats for diverse fungal communities through natural and human influences [13].
This study focused on investigating wood-decay fungi in Hongneung Forest, an urban forest in South Korea. In addition, the diversity of heart-rot fungi was analyzed.
In August and September 2021 (four times in total), we investigated wood-decay fungi inhabiting wood in Hongneung Forest, Seoul, South Korea. Samples were collected from three distinct sites: an arboretum, a landscape garden, and a foreign arboretum (Fig. 1).
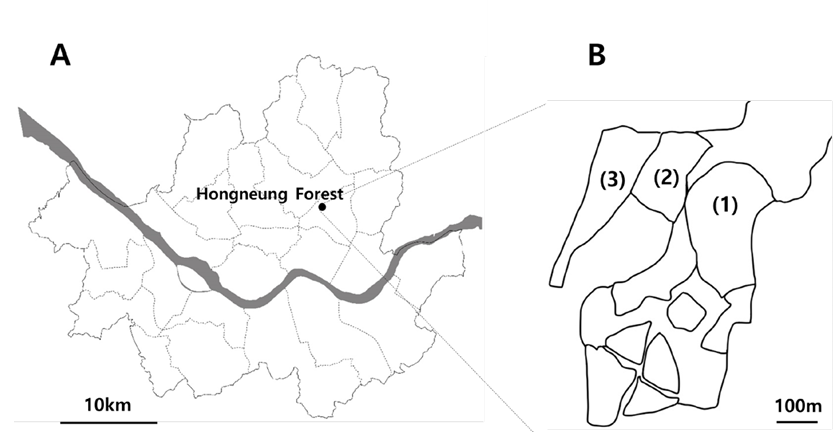
Fig. 1. Collection sites in Hongneung Forest. A, location of Hongneung Forest within Seoul; B, sampling sites. (1), arboretum; (2), landscape garden; (3), foreign arboretum.
Genomic DNA was extracted from samples collected in Hongneung Forest using a DNA Extraction Kit (Bioneer, Daejeon, Korea). The extracted genomic DNA was amplified using the internal transcribed spacer (ITS) region-specific primers ITS1F [14] and ITS4 [15]. Polymerase chain reaction (PCR) was performed using AccuPower PCR PreMix (Bioneer). The PCR conditions included a pre-denaturation step (94℃ for 5 min), followed by a second step involving 35 cycles (denaturation at 94℃ for 30 sec, annealing at 55℃ for 45 sec, extension at 72℃ for 45 sec), and a final extension step (72℃ for 7 min). PCR products were purified using an AccuPrep PCR Purification Kit (Bioneer, Daejeon, Korea). The DNA sequences of the PCR products were determined by Macrogen (Seoul, Korea).
Alignment analysis was performed using the ClustalW tool in MEGA X [16]. A phylogenetic tree was constructed using the Maximum Likelihood method with 1,000 bootstrap replicates. Reference ITS sequences of 32 wood decaying fungi were obtained from the National Center for Biotechnology Information.
Identification is primarily conducted using molecular techniques. The morphological characteristics were examined to confirm these results. They were analyzed based on field notes, photographs, and dried specimens. The macroscopic characteristics of the basidiocarps, such as shape, pileus surface, margin, and pore surface, were observed. For microscopic features, such as hyphae, cystidia, and basidiospores, slides were prepared in 3% KOH using an S8AP0 optical microscope (Leica Biosystems, Wetzlar, Germany) and observed using a DM2500 optical microscope (Leica Biosystems).
A total of 94 samples (42 from the arboretum, 38 from the landscape garden, and 14 from the foreign arboretum) were collected during the survey in Hongneung Forest. Morphological and molecular analyses identified 32 species, 26 genera, 18 families, and 11 orders (Table 1; Figs. 1 and 2). Among the identified species, 59% (19 species) belonged to the order Polyporales. The genera Perenniporia, Neohypochnicium, Coniophora, Ceratobasidium, and Scytinostroma exhibited the highest species richness. Of the 94 samples collected from the Hongneung Forest, 81% (26 species) were identified at the species level. The remaining samples could only be identified to the genus level or higher, owing to their indistinct morphological characteristics and the absence of matching sequences in GenBank. The dominance of Polyporales suggests that they play an important role in wood decomposition and nutrient cycling in Hongneung Forest. This is consistent with the results from other urban forests, where Polyporales play an important role in maintaining ecosystem functions [17].
Table 1. List of fungi collected from Hongeung Forest in Seoul
| Classification | Specimen | GenBank accession No. | Substrates | Site of occurrencea | |||
|---|---|---|---|---|---|---|---|
| Order or above | Family | Genus | Species | ITS | |||
| Fungi | |||||||
| Basidiomycota | |||||||
| Agaricomycetes | |||||||
| Agaricales | Strophariaceae | Gymnopilus | Gymnopilus junonius | NIFoS20210825-10 | LC852190 | – | 2 |
| Auriculariales | Auriculariaceae | Auricularia | Auricularia cornea | NIFoS20210825-02 | LC851593 | Sambucus williamsii | 2 |
| Boletales | Coniophoraceae | Coniophora | Coniophora arida | NIFoS20210826-09 | LC851564 | Pinus densiflora | 1 |
| NIFoS20210826-19 | LC851572 | Acer palmatum | 1 | ||||
| NIFoS20210825-06 | LC852186 | Viburnum wrightii | 2 | ||||
| NIFoS20210825-13 | LC852193 | Pinus densiflora | 2 | ||||
| Cantharellales | Ceratobasidiaceae | Ceratobasidium | Ceratobasidium sp. | NIFoS20210826-15 | LC851569 | – | 1 |
| NIFoS20210825-15 | LC852195 | Populus tremula | 2 | ||||
| NIFoS20210825-16 | LC852196 | Populus tremula | 2 | ||||
| NIFoS20210825-20 | LC852198 | Malus floribunda | 2 | ||||
| Hymenochaetales | Hymenochaetaceae | Fuscoporia | Fuscoporia gilvoides | NIFoS20210826-32 | LC851579 | Syringa reticulata | 1 |
| Phylloporia | Phylloporia fontanesiae | NIFoSXⅡ G-0274 | LC851587 | Fontanesia philliraeoides | 3 | ||
| Peniophorellaceae | Peniophorella | Peniophorella crystallifera | NIFoS20210826-17 | LC851571 | Quercus aliena | 1 | |
| Polyporales | Cerrenaceae | Cerrena | Cerrena unicolor | NIFoS20210826-37 | LC851583 | – | 1 |
| Somion | Somion delectans | NIFoS20210825-21 | LC852199 | Taxus cuspidata | 2 | ||
| Fomitopsidaceae | Bjerkandera | Bjerkandera adusta | NIFoS20210825-08 | LC852188 | Magnolia obovata | 2 | |
| Fomitopsis | Fomitopsis tropica | NIFoS20210825-05 | LC851596 | Prunus mume | 2 | ||
| Ganodermataceae | Ganoderma | Ganoderma gibbosum | NIFoS20210825-04 | LC851595 | Morus bombycis | 2 | |
| NIFoS20210825-11 | LC852191 | Prunus mume | 2 | ||||
| Ganoderma lucidum | NIFoS20210825-09 | LC852189 | Quercus aliena | 2 | |||
| Ganoderma sessile | NIFoSXⅡ G-0441 | LC851590 | Quercus serrata | 3 | |||
| Irpicaceae | Irpex | Irpex lacteus | NIFoSXⅡ G-0239 | LC851586 | Prunus padus | 3 | |
| Meripilaceae | Physisporinus | Physisporinus crataegi | NIFoS20210825-01 | LC851592 | Sambucus williamsii | 2 | |
| Neohypochniciaceae | Neohypochnicium | Neohypochnicium cremicolor | NIFoS20210826-13 | LC851567 | Prunus serrulata | 1 | |
| Neohypochnicium pini | NIFoS20210826-14 | LC851568 | Prunus serrulata | 1 | |||
| NIFoS20210826-33 | LC851580 | Castanea crenata | 1 | ||||
| NIFoS20210825-12 | LC852192 | Quercus serrata | 2 | ||||
| NIFoS20210825-26 | LC852200 | Taxus cuspidata | 2 | ||||
| Phanerochaetaceae | Phanerochaete | Phanerochaete cystidiata | NIFoS20210825-14 | LC852194 | Malus floribunda | 2 | |
| Polyporaceae | Coriolopsis | Coriolopsis strumosa | NIFoS20210826-30 | LC851577 | – | 1 | |
| Daedaleopsis | Daedaleopsis styracina | NIFoS20210825-17 | LC852197 | Styrax japonicus | 2 | ||
| Lopharia | Lopharia mirabilis | NIFoS20210825-03 | LC851594 | Magnolia kobus | 2 | ||
| Perenniporia | Perenniporia valliculorum | NIFoS20210826-26 | LC851574 | Quercus aliena | 1 | ||
| Perenniporia fraxinea | NIFoS20210826-16 | LC851570 | Zanthoxylum simulans | 1 | |||
| NIFoS20210826-34 | LC851581 | Quercus mongolica | 1 | ||||
| NIFoS20210826-39 | LC851585 | Prunus padus | 1 | ||||
| NIFoSXⅡ G-0423 | LC851589 | Prunus serotina | 3 | ||||
| NIFoSXⅡ G-0458 | LC851591 | Robinia pseudoacacia | 3 | ||||
| Trametes | Trametes coccinea | NIFoSXⅡ G-0415 | LC851588 | Prunus serotina | 3 | ||
| Trametes versicolor | NIFoS20210826-03 | LC851565 | Styphnolobium japonicum | 1 | |||
| Russulales | Lachnocladiaceae | Scytinostroma | Scytinostroma sp. | NIFoS20210826-28 | LC851575 | Prunus sargentii | 1 |
| NIFoS20210826-36 | LC851582 | Juniperus chinensis | 1 | ||||
| – | Baltazaria | Baltazaria sp. | NIFoS20210825-07 | LC852187 | Pinus densiflora | 2 | |
| NIFoS20210826-31 | LC851578 | Pinus densiflora | 1 | ||||
| Thelephorales | Thelephoraceae | Tomentella | Tomentella sp. | NIFoS20210826-12 | LC851566 | Quercus acutissima | 1 |
| Tomentella tedersooi | NIFoS20210826-29 | LC851576 | Sorbus alnifolia | 1 | |||
| Trechisporales | Hydnodontaceae | Trechispora | Trechispora sp. | NIFoS20210826-35 | LC851584 | Acer okamotoanum | 1 |
| Trechisporales | – | – | Trechisporales sp. | NIFoS20210826-20 | LC851573 | Prunus serrulata | 1 |
TS: internal transcribed spacer.
aThe numbers represent the three divided study segments in Hongeung Forest. 1, arboretum; 2, landscape garden; 3, foreign arboretum.
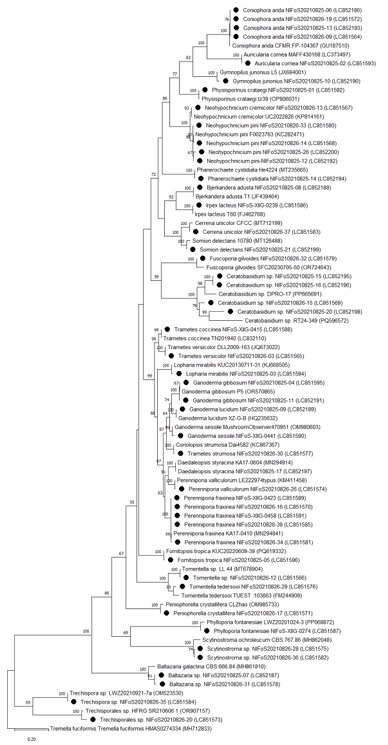
Fig. 2. Maximum likelihood phylogenetic tree of the wood-decay fungi in Hongneung Forest using internal transcribed spacer (ITS) region sequences. Bootstrap values >50% are shown. ●, samples collected in this study. Tremella fuciformis (MH712833) was used as an outgroup.
To the best of our knowledge, five species (Fomitopsis tropica, Ganoderma sessile, Peniophorella crystallifera, Perenniporia valliculorum, and Tomentella tedersooi) have not been reported previously in South Korea (Fig. 3). However, the microscopic features of the specimens, such as basidia and basidiospores, were not observed. Therefore, additional sampling will be required to confirm their presence in South Korea.
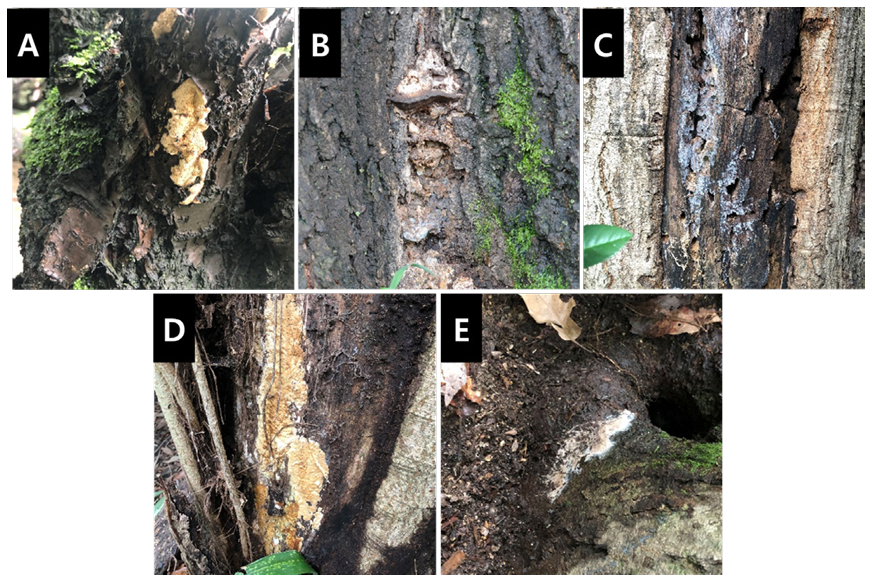
Fig. 3. Wood-decay fungi that were not previously recorded in Korea. A, Fomitopsis tropica; B, Ganoderma sessile; C, Peniophorella crystallifera; D, Perenniporia valliculorum; E, Tomentella tedersooi.
Heart-rot fungi are pathogenic organisms that contribute to wood-decay in urban forests. Among the 32 species sampled from Hongneung Forest, Bjerkandera adusta, Coniophora arida, Perenniporia fraxinea, and Somion delectans have been reported as heart-rot fungi (Fig. 4) [5,18,19]. P. fraxinea was observed in five species (Prunus padus, P. serotina, Quercus mongolica, Robinia pseudoacacia, and Zanthoxylum simulan). C. arida was observed in three species (Acer palmatum, Pinus densiflora, and Viburnum wrightii). B. adusta, and S. delectans were detected on Magnolia obovata, and Taxus cuspidata, respectively. Trees such as T. cuspidata, P. densiflora, and Q. mongolica are widely distributed in Korea [20,21].
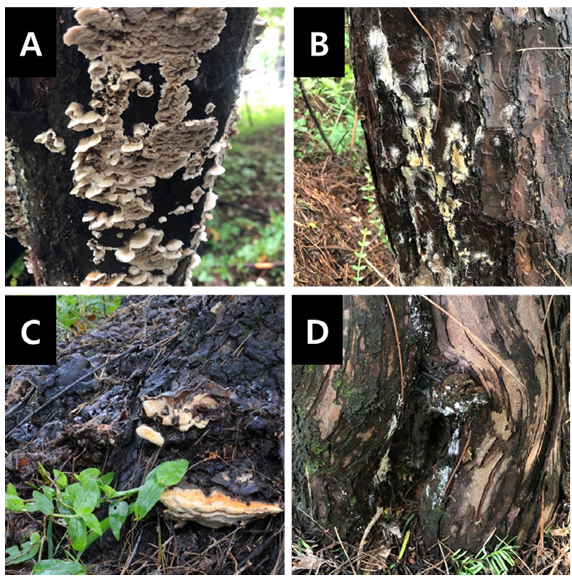
Fig. 4. Heart-rot fungi observed in Hongneung Forest, Seoul, Korea. A, Bjerkandera adusta; B, Coniophora arida; C, Perenniporia fraxinea; D, Somion delectans.
B. adusta, which causes white rot, was found on the tree trunks. C. arida exhibits cream-to-buff basidiocarps on the pine bark, which causes internal decay. P. fraxinea was predominantly found at the bases of the trees, and the health of the infected trees was weakened. S. delectans caused white rot on the lower parts of the tree, but no basidiocarps were observed. Severely infected trees exhibit symptoms, such as reduced leaf density and thinning canopies [7].
To examine intraspecific variations, nucleotide-level ITS sequences were compared among four specimens of C. arida (NIFoS20210825-06, NIFoS20210825-13, NIFoS20210826-09, and NIFoS20210826-19) and five specimens of P. fraxinea (NIFoS-XIIG-0423, NIFoS-XIIG-0458, NIFoS20210826-16, NIFoS20210826-34, and NIFoS20210826-39). C. arida showed two variants-a difference at the 52nd nucleotide in ʻNIFoS20210825—13’ (Fig. 5A). In terms of P. fraxinea, there were four variants—ʻNIFoS20210826-16’ differed at the 70th nucleotide from ʻNIFoS-XIIG-0423’ and ʻNIFoS-XIIG-0458’. ʻNIFoS20210826-34’ showed differences at the 57th and 92nd nucleotides, and ʻNIFoS20210826-39’ showed differences at the 85th and 571st nucleotides (Fig. 5B).
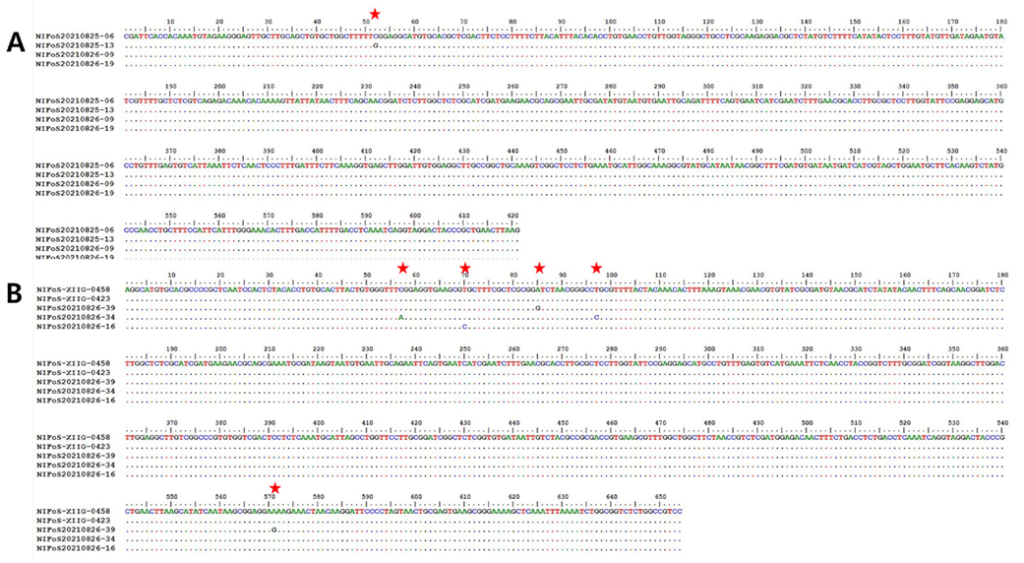
Fig. 5. Comparative analysis of internal transcribed spacer (ITS) sequences at the nucleotide level within the same species of A, Coniophora arida; and B, Perenniporia fraxinea. Dots indicate the same nucleotides as shown in the first sequence.
Determining how the heart-rot fungi identified here infected the trees is difficult. Various possible routes of infection exist, such as entering through peeled bark or being transported by insects [22]. In addition, we attempted to isolate fungi from the specimens to determine their pathogenicity; however, this was unsuccessful. Further research is needed to understand infection and pathogenicity in the host. This study provides information on the diversity of wood-decay fungi in the Hongneung Forest, an urban forest in South Korea, and is expected to serve as basic data for future research on heart-rot fungi in Korean forests.
There are no relevant financial or non-financial competing interests to report.
This study was supported by a grant from the General Project (FP0800-2023-01) of the National Institute of Forest Science of the Republic of Korea.
1. Yoo RH, Lee KS, Kim SK, Bae SW, Yoon EY. A study on the preference analysis by use type of urban forests. J Korean Inst For Recreat 2007;11:1-6.
2. Kim HH, Lee JE, Lee SY, Park DE, Yun CW. Vegetation structure of urban forests on Mt. Goehwa, Sejong-si. J Korean Soc For Sci 2024;113:51-65.
3. Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010;468:647-52. [DOI]
4. Balla A, Silini A, Cherif-Silini H, Bouket AC, Moser WK, Nowakowska JA, Oszako T, Benia F, Belbahri L. The threat of pests and pathogens and the potential for biological control in forest ecosystems. Forests 2021;12:1579. [DOI]
5. Dai YC, Cui BK, Yuan HS, Li BD. Pathogenic wood-decaying fungi in China. For Pathol 2007:37:105-20. [DOI]
6. Sinclair W, Lyon HH. Diseases of trees and shrubs, 2nd ed. Ithaca, New York: Cornell University Press; 2005. p. 660.
7. Jha SK. Identification and management of heart-rot fungi. Banko Janakari 2020;30:71-7. [DOI]
8. Schmidt O, Gaiser O, Dujesiefken D. Molecular identification of decay fungi in the wood of urban trees. Eur J Forest Res 2012;131:885-91. [DOI]
9. Song IJ, Yoon CR. Development and application of biodiversity indicator in parks and green spaces in Seoul. Seoul: The Seoul Institute; 2018.
10. Kang S, Kim JO. Morphological analysis of green infrastructure in the Seoul metropolitan area, South Korea. Landscape Ecol Eng 2015;11:259-68. [DOI]
11. Choi GY, Kim T. A study on the management plan of Hongneung Forest based on visitor monitoring. J Korean Soc For Sci 2015;104:443-53. [DOI]
12. Jo JH, Park CY, Kim SK. Hongneung forest story. Seoul: National Institute of Forest Science; 2011. p. 4.
13. Scholier T, Lavrinienko A, Brila I, Tukalenko E, Hindström R, Vasylenko A, Cayol C, Ecke F, Singh NJ, Forsman JT, et al. Urban forest soils harbour distinct and more diverse communities of bacteria and fungi compared to less disturbed forest soils. Mol Ecol 2023;32:504-17. [DOI]
14. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8. [DOI]
15. White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, editors. PCR protocols: A guide to methods and applications. New York: Academic Press; 1990. p. 315-22. [DOI]
16. Kumar S, Tamura K, Nei M. MEGA: Molecular evolutionary genetics analysis software for microcomputers. Bioinformatics 1994;10:189-91. [DOI]
17. Korhonen A, Penttilä R, Siitonen J, Miettinen O, Immonen A, Hamberg L. Urban forests host rich polypore assemblages in a Nordic metropolitan area. Landsc Urban Plan 2021;215:104222. [DOI]
18. Norokorpi Y. Old Norway spruce stands, amount of decay and decay-causing microbes in northern Finland. Helsinki: Communicationes Instituti Forestalis Fenniae; 1979.
19. Kehr R, Dujesiefken D, Wohlers ANTJE, Wulf A. Root and butt decay of Robinia pseudoacacia caused by Perenniporia fraxinea. In: Proceeding of International Symposium on Plant Health in Urban Horticulture; 2000 May 22-25; Braunschweig, Germany. Braunschweig: Biologische Bundesanstalt für Land- und Forstwirtschaft in Berlin und Braunschweig; 2000. p. 92-6.
20. Kim J, Lee DK, Kim HG. Suitable trees for urban landscapes in the Republic of Korea under climate change. Landsc Urban Plan 2020;204:103937. [DOI]
21. Han BH, Park SC, Kim JY, Kwak JI. Ecological characteristics and changes of Quercus mongolica community in Namsan (Mt.), Seoul. J Korean Inst Landsc Archit 2022;50:41-63. [DOI]
22. Boddy L. Latent decay fungi: the hidden foe?. Arboric J 1994;18:113-35. [DOI]