Min Kyung Kim1, Hong Hee Jeong2, Dong Hyun Son3, Geon Sik Seo1, and Myung Soo Park1*
1Department of Mushroom Science, Korea National University of Agriculture and Fisheries, Jeonju 54874, Korea
2Mush&, Jeonju 54874, Korea
3Dongguri-Farm, Jinan, 55414, Korea
*Correspondence to mshy1219@af.ac.kr
Korean Journal of Mycology (Kor J Mycol) 2025 September, Volume 53, Issue 3, pages 183-192.
https://doi.org/10.4489/kjm.2025.53.3.5
Received on September 01, 2025, Revised on September 08, 2025, Accepted on September 09, 2025, Published on September 30, 2025.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-Non-Commercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Reishi is a medicinal mushroom widely cultivated in East Asia and is valued for its bioactive compounds. However, fungal contamination during postharvest storage can cause discoloration and mycotoxin contamination, threatening product safety and quality. Despite its economic importance, fungal communities associated with stored dried Reishi have not been studied in Korea. In this study, 35 fungal strains were isolated from stored dried Reishi collected in Korea. Based on internal transcribed spacer regions, β-tubulin, and calmodulin, the strains were identified as 17 species within five genera. Aspergillus and Penicillium were predominant, with A. chevalieri, A. montevidensis, A. fumigatus, Coniochaeta velutina, and P. citrinum being the most frequently detected species. Several Aspergillus and Penicillium species detected in this study are known producers of mycotoxins, indicating potential risks to product safety. This study is the first to examine the fungal communities associated with stored dried Reishi in Korea. These results suggest the importance of effective postharvest management practices to control contamination and maintain the safety and quality of Reishi products. In addition, detailed morphological descriptions of Talaromyces macrosporus, a previously unrecorded species, are provided.
Aspergillus, Penicillium, Reishi, Talaromyces macrosporus, Unrecorded species
Reishi (Ganoderma sichuanense) is a medicinal mushroom that has been valued for centuries in East Asia, including China, Korea, and Japan. Across different regions of the world, it is referred to by various synonyms and names reflecting its long history of traditional use [1]. Owing to its various bioactive compounds, Reishi has been widely used in the development of cosmetics, health supplements, and functional foods [1,2]. Therefore, it is one of the most extensively cultivated and consumed medicinal mushrooms worldwide.
In recent years, fungal diseases have emerged as a major concern in the commercial cultivation and postharvest management of Reishi. Several fungal pathogens have been reported during cultivation, including cobweb disease caused by Cladobotryum mycophilum [3], green mold disease caused by Trichoderma spp. [4,5], and yellow rot caused by Scytalidium ganodermophthorum [6]. Postharvest changes in environmental conditions cause a shift in the fungal community as field-derived fungi are gradually replaced by fungi associated with postharvest diseases, particularly Aspergillus and Penicillium, which can lead to discoloration, loss of dry matter, and contamination of dried mushrooms with mycotoxins [7–9]. Aspergillus flavus, A. ochraceus, and Penicillium citrinum have frequently been reported as contaminants in stored Reishi [8,10], posing a potential risk to both product quality and consumer safety.
Despite the clear importance of postharvest management, fungal communities associated with stored dried Reishi have not yet been studied in Korea. Therefore, the aim of this study was to explore the fungal diversity in stored dried Reishi. In particular, Talaromyces macrosporus, a previously unrecorded species, was identified through phylogenetic analysis of the β-tubulin (BenA) and calmodulin (CaM) sequences, together with comprehensive morphological characterization.
Fungal-contaminated samples were collected from dried Reishi stored at Dongguri Farm, Jinan, on June 23, 2023 (Fig. 1). The samples were cut into discs of approximately 5 mm in diameter and transferred to dichloran rose bengal chloramphenicol agar (DRBC agar; Difco, Becton Dickinson, MD, USA). After incubation at 25°C for 7 days, fungal mycelia grown from the discs were subsequently transferred to potato dextrose agar (PDA; Difco, Becton Dickinson, MD, USA) for isolation. The purified cultures were preserved in 20% glycerol at -80°C in the culture collection of the Korea National University of Agriculture and Fisheries.
The obtained sequences were assembled, manually corrected using MEGA5 [12] and submitted to GenBank (accession numbers are listed in Table 1). Multiple alignments were generated using MAFFT v7 [13]. Pairwise sequence similarities of unrecorded species were calculated for both loci using MEGA5 [12]. Phylogenetic analysis was performed with RAxML [14] using the maximum likelihood approach under the General Time Reversible (GTR) + GAMMA model with 1,000 bootstrap replicates performed on CIPRES web platform [15].
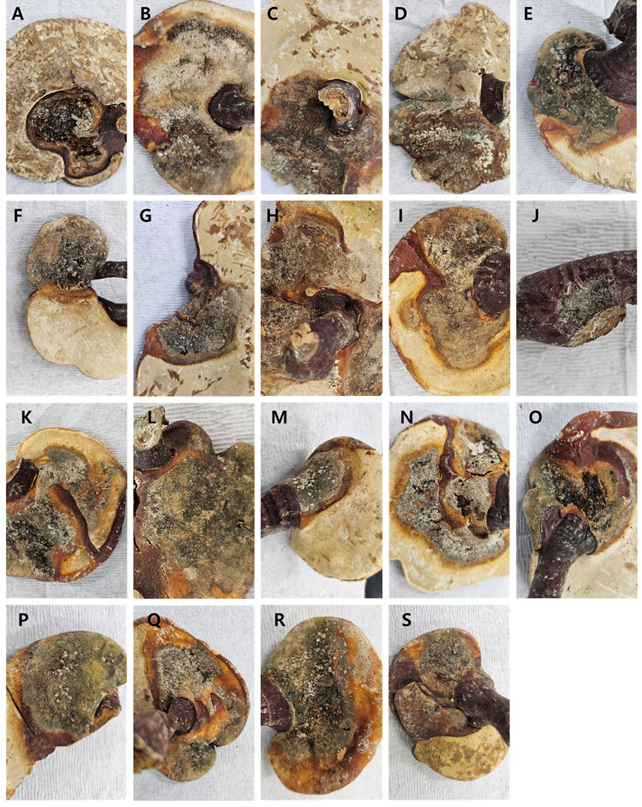
Fig. 1. Stored dried Reishi contaminated with fungi.
The morphological characteristics of the unrecorded species were examined using standard procedures [16] with four different media: Czapek yeast autolysate agar (CYA), malt extract agar (MEA), yeast extract sucrose agar (YES), and oatmeal agar (OA). Colony colors were described using color names and codes from the Methuen Handbook of Color [17]. The micromorphological characters were observed from 7-dayold MEA culture incubated 25°C, using Leica DM2000 light microscope (Leica Microsystems, Wetzlar, Germany).
Table 1. GenBank accession numbers of fungal strains isolated from stored dried Reishi
테이블
ITS, internal transcribed spacer region, BenA: β-tubulin, CaM: calmodulin.
A total of 35 fungal strains were isolated from stored dried Reishi. Based on sequence analysis of the ITS regions, BenA, and CaM, these strains were identified as 17 species within five genera, including one previously unrecorded species (MF0442-1) (Table 1).
At the genus level, 62.8% of the species (n = 22) belonged to Aspergillus, 14.3% belonged to Penicillium (n = 5), 8.6% belonged to Coniochaeta (n = 3), 8.6% belonged to Talaromyces (n = 3), and 5.7% belonged to Scytalidium (n = 2) (Fig. 2A). The dominant species were A. chevalieri (n = 7) and A. montevidensis (n = 5), followed by A. fumigatus (n = 3), C. velutina (n = 3), and P. citrinum (n = 3) (Fig. 2B). Each contaminated sample contained one to four species (Table 1).
The previously unrecorded strain MF0442-1 (NIBRFGC000510857) was reconfirmed using concatenated dataset of BenA and CaM gene sequences. MF0442-1 grouped with the type strain (CBS 317.63) of T. macrosporus (bootstrap support = 100%; sequence similarity for BenA = 100% and CaM = 100%) (Fig. 3).
Talaromyces macrosporus (Stolk & Samson) Frisvad, Samson & Stolk, Antonie van Leeuwenhoek 57: 186. 1990. MycoBank MB126704.
Colony growth (7 days, diameter in mm): CYA at 25°C: 28–30, at 30°C: 40–42, and at 37°C: 30–35; MEA at 25°C: 46–47; and YES at 25°C: 40–42 (Fig. 4).
Colony characteristics: On CYA at 25°C for 7 days, colonies were slightly elevated at center with entire margins. The mycelia appeared white, orange-white (5A2), and grayish-orange (5B3). The texture was f loccose, with poor sporulation. No exudate was produced. The soluble pigment was orange-white (5A2). The reverse color was dark brown (6F6). On MEA at 25°C for 7 days, colonies were flat with entire margins and formation of pale yellow (3A3) ascomata at center. The mycelia appeared white and reddishwhite (7A2). Pale yellow (3A3) ascomata formed at the center. The texture was floccose, with sparse sporulation. Exudates of brown droplets were produced at the center. The soluble pigment was grayishorange (5B4). The reverse color was light brown (6D6). On YES at 25°C for 7 days, colonies were low and sulcate with entire margins. The mycelia were orange-white (5A2) and pale yellow (4A3), with a floccose texture. Sporulation is sparse. Exudates of small, clear droplets were also observed. Soluble pigments grayish orange (5B4). The reverse colors were brownish-orange (5C5) and brown (6E6).
Conidiophores were monoverticillate and biverticillate with smooth stipes. Phialides were ampulliform, 8–12(–15) × 2–3 μm. Conidia were smooth-walled, subglobose to ellipsoidal, 2–3 × 2–2.5 μm diam.
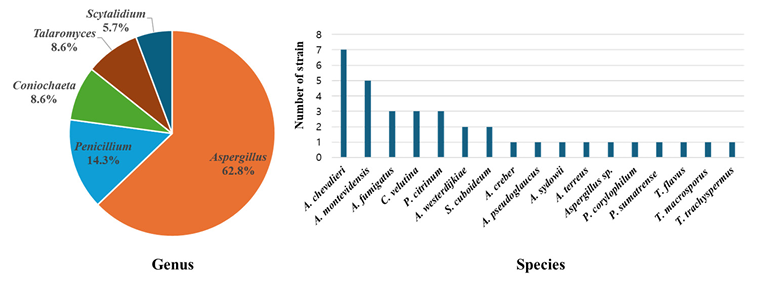
Fig. 2. Composition of fungal strains isolated from stored dried Reishi at the genus level (A) and species level (B).
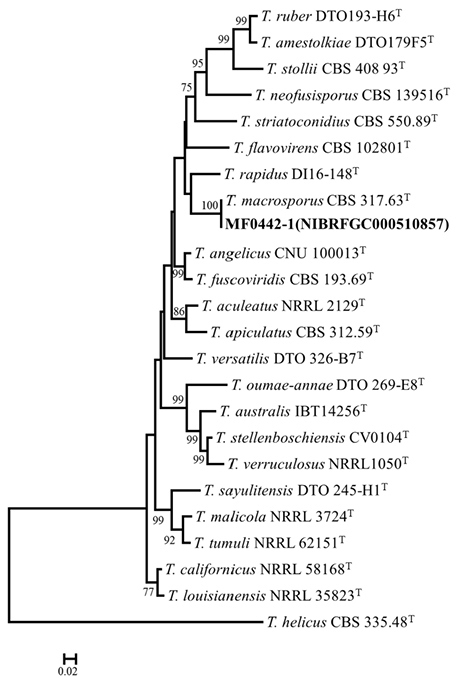
Fig. 3. Maximum likelihood phylogenetic tree based on the combined dataset of β-tubulin (BenA) and calmodulin (CaM) gene sequence used to identify Talaromyces strains from stored dried Reishi. Bootstrap scores of >70 are presented at the nodes. The scale bar indicates the number of nucleotide substitutions per site. “T” indicates the ex-type strains. Strains reported in the current study are represented in bold.
Ascomata formed abundantly within 10 days on OA at 25°C, globose to subglobose, 100–450 × 100–400 μm, yellow in color. Asci measured 13–16 × 11–13 μm. Ascospores were subglobose to broadly ellipsoidal, thick-walled, ornamented with spiny, 5–6 × 4.0–5.5 μm.
Strain examined: MF0442-1 (NIBRFGC000510857)
Note: Talaromyces macrosporus shares morphological features, such as ascomata and rapid growth, with T. muroii and T. liani, but is clearly distinguished from them in phylogenetic analyses. The Korean isolate of T. macrosporus exhibited a higher growth rate compared with the type strain CBS 317.63.
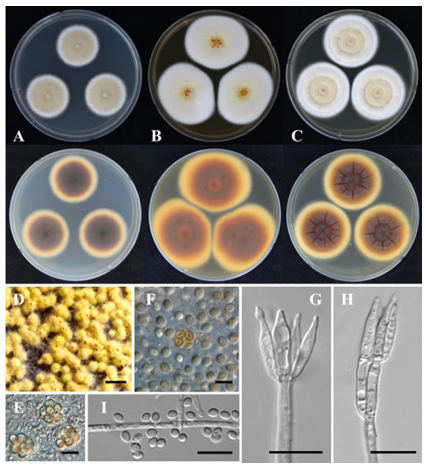
Fig. 4. Talaromyces macrosporus MF0442-1 (NIBRFGC000510857) in 7-day-old cultures at 25°C. AC: Colonies grown on Czapek yeast autolysate agar (CYA), malt extract agar (MEA), and yeast extract sucrose agar (YES) from left to right (top = obverse, bottom = reverse). D: Ascomata on oatmeal agar (OA) after 2 weeks of incubation. E, F: Asci and ascospores. G, H: Conidiophores. I: Conidia. Scale bars: D, 1,000 μm; E–I, 10 μm.
Edible and medicinal mushrooms are vulnerable to fungal contamination during harvesting, processing, and storage. Such contamination can lead to discoloration, nutrient degradation, and mycotoxin production [7,8]. We isolated 35 fungal strains from stored dried Reishi and identified 17 species belonging to five genera. Importantly, one previously unrecorded species was confirmed based on multilocus sequence analysis (BenA and CaM) and morphological characteristics. Among the isolates, Aspergillus and Penicillium were the most dominant genera, indicating that these genera play a central role in shaping the fungal community of stored dried Reishi. These results are consistent with previous reports that fungi associated with postharvest diseases frequently colonize mushrooms, with Aspergillus and Penicillium known to be the major contaminants [7–9].
Within the genus Aspergillus, A. chevalieri, A. montevidensis, and A. fumigatus are among the most frequently isolated species. Members of this genus can tolerate low water activity and high osmotic pressure, which are commonly encountered in dried and semi-dried mushroom products [9,18]. Aspergillus chevalieri, A. fumigatus, A. terreus, and A. westerdijkiae detected in this study are known to produce secondary metabolites such as aflatoxins and ochratoxins [19]. Penicillium citrinum was one of the most frequently isolated species in this study and is known to produce citrinin and other mycotoxins [20]. These results show that Aspergillus and Penicillium are the predominant storage fungi in Reishi and play major roles in mycotoxin contamination.
In addition to the dominant genera, Coniochaeta velutina and S. cuboideum have been isolated from stored Reishi [21,22]. These species are associated with wood staining and are likely to contribute to surface discoloration and reduce product quality during storage. The detection of T. macrosporus in stored dried Reishi is particularly interesting. Although phylogenetic analysis confirmed its identity to the type strain, the Korean isolate showed slight morphological differences, such as faster growth, which may indicate ecological adaptation to storage conditions [23]. Talaromyces macrosporus produces heat-resistant, dormant ascospores that can survive under extreme environment [24,25], its detection in stored dried Reishi suggests a possible role in long-term persistence and product quality decline during storage.
This is the first comprehensive investigation of fungi associated with stored dried Reishi in Korea. These results reveal that careful monitoring and effective postharvest management are important for preventing fungal contamination, lowering the risk of mycotoxin production, and ensuring the safety and quality of Reishi products.
The authors declare no conflict of interest.
This work was supported by the Basic Science Research Program (No. 2019R1I1A1A01061954) through the National Research Foundation of Korea (NRF), funded by the Ministry of Education and National Institute of Biological Resources (NIBR202002104 and NIBR202511101) under the Ministry of Environment, Republic of Korea.
1. Ahmad R, Riaz M, Khan A, Aljamea A, Algheryafi M, Sewaket D, Alqathama A. Ganoderma lucidum (Reishi) an edible mushroom; A comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother Res 2021;35:6030-62. https://doi.org/10.1002/ptr.7215 [DOI]
2. Li S, Dong C, Wen HA, Liu X. Development of Ling-zhi industry in China-emanated from the artificial cultivation in the Institute of Microbiology, Chinese Academy of Sciences (IMCAS). Mycology 2016;7:74-80. https://doi.org/10.1080/2150120 [DOI]
3.2016.1171805 3. Zuo B, Lu BH, Liu XL, Wang Y, Ma GL, Gao J. First report of Cladobotryum mycophilum causing cobweb on Ganoderma lucidum cultivated in Jilin province, China. Plant Dis 2016;100:1239. https://doi.org/10.1094/PDIS-12-15-1431-PDN [DOI]
4. Cai M, Idrees M, Zhou Y, Zhang C, Xu J. First report of green mold disease caused by Trichoderma hengshanicum on Ganoderma lingzhi. Mycobiology 2020;48:427-30. https://doi. org/10.1080/12298093.2020.1794230 [DOI]
5. An XY, Cheng GH, Gao HX, Li XF, Yang Y, Li D, Li Y. Phylogenetic analysis of Trichoderma species associated with green mold disease on mushrooms and two new pathogens on Ganoderma sichuanense. J Fungi 2022;8:704. https://doi.org/10.3390/jof8070704 [DOI]
6. Kang HJ, Sigler L, Lee J, Gibas CFC, Yun SH, Lee YW. Xylogone ganodermophthora sp. nov., an ascomycetous pathogen causing yellow rot on cultivated mushroom Ganoderma lucidum in Korea. Mycologia 2010;102:1167-84. https://doi.org/10.3852/09-304 [DOI]
7. Chen L, Wu J, Zhang S, Liu X, Zhao M, Guo W, Zhang J, Chen W, Liu Z, Deng M, et al. Occurrence and diversity of fungi and their mycotoxin production in common edible and medicinal substances from China. J Fungi 2025;11:212. https://doi.org/10.3390/jof11030212 [DOI]
8. Ezekiel CN, Sulyok M, Frisvad JC, Somorin YM, Warth B, Houbraken J, Samson RA, Krska R, Odebode AC. Fungal and mycotoxin assessment of dried edible mushroom in Nigeria. Int J Food Microbiol 2013;162:231-6. https://doi.org/10.1016/j.ijfoodmicro.2013.01.025 [DOI]
9. Pitt JI, Hocking AD. Fungi and food spoilage. 3rd ed. New York, NY: Springer; 2009. p. 388. [DOI]
10. Chen L, Guo W, Zheng Y, Zhou J, Liu T, Chen W, Liang D, Zhao M, Zhu Y, Wu Q, et al. Occurrence and characterization of fungi and mycotoxins in contaminated medicinal herbs. Toxins 2020;12:30. https://doi.org/10.3390/toxins12010030 [DOI]
11. Park MS, Fong JJ, Oh SY, Houbraken J, Sohn JH, Hong SB, Lim YW. Penicillium jejuense sp. nov., isolated from the marine environments of Jeju Island, Korea. Mycologia 2015;107:20916. https://doi.org/10.3852/14-180 [DOI]
12. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731-9. https://doi.org/10.1093/ molbev/msr121 [DOI]
13. Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol 2013;30:772-80. https://doi. org/10.1093/molbev/mst010 [DOI]
14. Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006;22:2688-90. https://doi. org/10.1093/bioinformatics/btl446 [DOI]
15. Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES science gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE); 2010 Nov 13-19; New Orleans, LA. Los Alamitos, CA: IEEE Computer Society; 2010. p. 1-8. [DOI]
16. Visagie CM, Houbraken J, Frisvad JC, Hong SB, Klaassen CHW, Perrone G, Seifert KA, Varga J, Yaguchi T, Samson RA. Identification and nomenclature of the genus Penicillium. Stud Mycol 2014;78:343-71. https://doi.org/10.1016/j.simyco.2014.09.001 [DOI]
17. Kornerup A, Wanscher JH. Methuen handbook of colour. 3rd ed. London: Methuen Publishing; 1978.
18. Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, Rediers H, Lievens B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb Biotechnol 2016;10:296-322. https:// doi.org/10.1111/1751-7915.12367 [DOI]
19. Navale V, Vamkudoth KR, Ajmera S, Dhuri V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol Rep 2021;8:1008-30. https://doi. org/10.1016/j.toxrep.2021.04.013 [DOI]
20. Houbraken JAMP, Frisvad JC, Samson RA. Taxonomy of Penicillium citrinum and related species. Fungal Divers 2010;44:117-33. https://doi.org/10.1007/s13225-010-0047-z [DOI]
21. Diplock ND, Galea VJ, Dorji, Norbu, Watanabe K, Terashima Y. Scytalidium cuboideum inhibits shiitake mycelial growth and causes pink staining in shiitake billets (Quercus griff ithii) in Bhutan. Int J Plant Biol 2023;14:949-58. https://doi.org/10.3390/ijpb14040069 [DOI]
22. Kim MJ, Choi YS, Oh JJ, Kim GH. Experimental investigation of the humidity effect on wood discoloration by selected mold and stain fungi for a proper conservation of wooden cultural heritages. J Wood Sci 2020;66:31. https://doi.org/10.1186/s10086-020-01878-z [DOI]
23. Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol 2014;78:175-341. https://doi.org/10.1016/ j.simyco.2014.08.001 [DOI]
24. Dijksterhuis J. Heat-resistant ascospores. In: Dijksterhuis J, Samson RA, editors. Food mycology. Boca Raton, FL: CRC Press; 2007. p. 115-32. [DOI]
25. Dijksterhuis J, Teunissen PGM. Dormant ascospores of Talaromyces macrosporus are activated to germinate after treatment with ultra high pressure. J Appl Microbiol 2004;96: 162-9. https://doi.org/10.1046/j.1365-2672.2003.02133.x [DOI]