Wan-Gyu Kim1,*, Gyo-Bin Lee1, Sung-Kee Hong2, Young-Moon Mo3, and Weon-Dae Cho1
1Global Agro-Consulting Corporation, Suwon 16614, Korea
2Crop Protection Division, National Institute of Agricultural Sciences, Wanju 55365, Korea
3Crop Research Division, Gangwon State Agricultural Research and Extension Services, Chuncheon 24203, Korea
*Correspondence to wgkim5121@naver.com
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 233-237.
https://doi.org/10.4489/kjm.520401
Received on September 02, 2024, Revised on November 20, 2024, Accepted on November 20, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Anthracnose symptoms were observed on the leaves of Korean epimedium (Epimedium koreanum) plants grown in two fields in Cheolwon, Korea, during disease surveys conducted in September 2019 and 2020. The symptoms were characterized by brown to dark brown, small spots with circular or irregular shapes on the leaves in the early stage. In the later stage, the lesions expanded to 5–10 mm in diameter. The proportion of diseased leaves in the surveyed fields ranged from 10% to 30%. Five single-conidium isolates of Colletotrichum sp. were obtained from the lesions and tested for pathogenicity on Korean epimedium leaves via artificial inoculation. Among the five isolates, only two were pathogenic to the host plant. Anthracnose symptoms induced by these isolates were similar to those observed in the surveyed fields. The Colletotrichum sp. isolates were identified as Colletotrichum gloeosporioides based on their morphological characteristics and phylogenetic analysis. To the best of our knowledge, this is the first report of C. gloeosporioides causing anthracnose in Korean epimedium.
Anthracnose, Colletotrichum gloeosporioides, Epimedium koreanum, Korean epimedium
The Korean epimedium (Epimedium koreanum Nakai) belongs to the family Berberidaceae. This plant grows primarily in temperate biomes and is native to southeast China, Japan, Korea, Manchuria, and Primorye [1]. It is a perennial species that has been cultivated for medicinal purposes in Korea.
In September 2019 and 2020, anthracnose symptoms were observed on the leaves of Korean epimedium plants grown in two fields in Cheolwon, Korea, during disease surveys for medicinal crops. The symptoms were characterized by brown to dark brown, small spots with circular or irregular shapes on the leaves in the early stage. In the later stage, the lesions expanded to 5–10 mm in diameter (Fig. 1A and 1B). Severely infected leaves were occasionally perforated. The proportion of diseased leaves in the surveyed fields ranged from 10% to 30%.
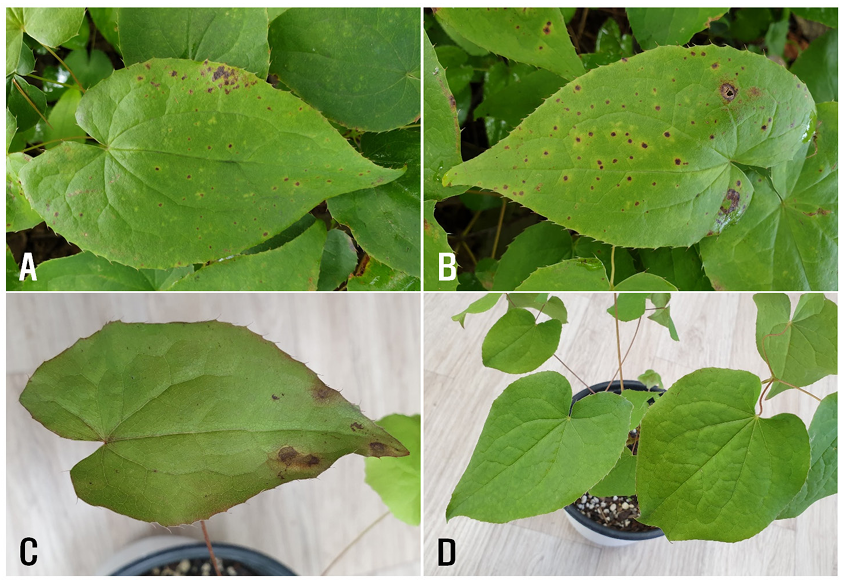
Fig. 1. Anthracnose symptoms of Korean epimedium plants. A and B: Symptoms observed on leaves in the field. C: Symptoms induced on the leaves by artificial inoculation with Colletotrichum sp. isolate (EKCO-04). D: A non-inoculated plant (control).
We collected diseased leaves of Korean epimedium from the surveyed fields and isolated fungi from the lesions. Lesion pieces, 3–5 mm in length, were excised from the infected leaves and plated on 2% water agar (WA) after surface sterilization with 1% sodium hypochlorite solution for one minute. The WA plates with lesion pieces were incubated at 25℃ for seven days. Conidial masses, produced on the lesions, were then used to prepare conidial suspension in sterile distilled water. The conidial suspension was streaked onto WA plates using a sterile loop. After incubating the plates at 25℃ for one day, germinated conidia were transferred to new WA plates. Single-conidium isolates were obtained from the mycelia growing on these new WA plates after incubating at 25℃ for five days. Five single-conidium isolates were obtained from the lesions and morphologically identified as Colletotrichum sp. based on descriptions in the previous studies [2,3].
Two-year-old Korean epimedium plants grown in circular plastic pots (height, 15 cm; upper diameter, 17 cm; lower diameter, 10 cm) in a vinyl greenhouse were used for pathogenicity tests. The five isolates were cultured on V8 juice agar (V8A) at 25℃ under fluorescent light for 23 days. Conidial suspensions (1-3 × 106 conidia/mL) were prepared from the V8A cultures. Twenty milliliters of each conidial suspension were sprayed on the leaves of Korean epimedium plants in a circular plastic pot. Control plants were sprayed with an equal volume of sterile distilled water. The inoculated and control plant pots were placed in plastic boxes (71.0 × 53.5 × 40.5 cm) under 100% relative humidity in a room maintained at 24-26℃. After five days, the pots were removed from the plastic boxes and kept in the room. The inoculation tests were performed in triplicate. Pathogenicity of the isolates was assessed based on the occurrence of anthracnose symptoms 12 days after inoculation. Among the five isolates tested, only two (EKCO-04 and EKCO-07) exhibited pathogenicity on the leaves of the host plant (Table 1). Anthracnose symptoms induced by the isolates were observed on the leaves of inoculated plants (Fig. 1C), whereas no symptoms were observed in he control plants (Fig. 1D). The anthracnose symptoms on the leaves of inoculated plants resembled those observed in the surveyed fields. The same fungus was re-isolated from the induced lesions.
Table 1. Conidial shapes of Colletotrichum sp. isolates from Korean epimedium leaves and pathogenicity of the isolates on the host plant
| Isolate | Date isolated | Conidial shape | Pathogenicitya |
|---|---|---|---|
| EKCO-01 | September 15, 2019 | Straight, fusiform | − |
| EKCO-04 | September 15, 2019 | Straight, cylindrical | + |
| EKCO-07 | September 15, 2019 | Straight, cylindrical | + |
| EKCO-13 | September 7, 2020 | Falcate, fusiform | − |
| EKCO-15 | September 7, 2020 | Falcate, fusiform | − |
aPathogenicity test was conducted via artificial inoculation of the isolates onto the leaves of 2-year-old Korean epimedium plants grown in plastic pots in a vinyl greenhouse. +, pathogenic; −, non-pathogenic.
The morphological characteristics of the two isolates pathogenic to Korean epimedium leaves were investigated. The morphology of 25 conidia from each isolate, produced in the V8A culture, was examined using a light microscope (Nikon Eclipse Ci-L, Tokyo, Japan). The conidia were straight or cylindrical, measuring 13.4-20.8 × 3.4-6.0 μm (average 16.7 × 4.8 μm) (Fig. 2A). A 20 µL conidial suspension (3-5 × 106 conidia/mL) of each isolate, prepared from V8A culture, was placed on WA, covered with a sterile cover glass, and incubated at 25℃ for one day. The morphology of the 25 appressoria from each isolate, produced in WA culture, was then examined using a light microscope. The appressoria were clavate, ovate, obovate, occasionally lobed, and measured 7.0-10.7 × 5.0-8.6 μm (average 8.4 × 6.7 μm) (Fig. 2B).
The morphological characteristics of the conidia and appressoria were similar to those of Colletotrichum gloeosporioides (Penz.) Penz. & Sacc., as described in previous studies [2,3].
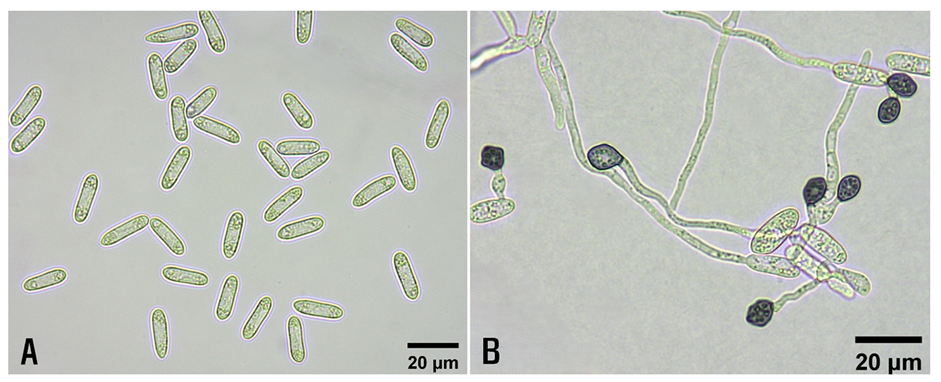
Fig. 2. Morphological features of Colletotrichum gloeosporioides isolates from diseased Korean epimedium plants. A: Conidia. B: Appressoria.
To confirm the identification of the isolates, phylogenetic analysis was performed. Genomic DNA was extracted from potato dextrose agar cultures grown at 25°C in the dark for seven days using the Maxwell® RSC PureFood GMO and Authentication Kit (Promega, Madison, USA). Four gene regions were targeted: 5.8S nuclear ribosomal gene with its two flanking internal transcribed spacers (ITS), partial sequences of the glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH), actin gene (ACT), and β-tubulin gene (TUB2). These regions were amplified and sequenced using ITS1/ITS4, GDF1/GDR1, ACT-512F/ACT-783R, and BT2Fd/BT4Rd primer sets, respectively [4-7]. The polymerase chain reaction (PCR) reactions were performed in a total volume of 20μL, containing 50 ng of genomic DNA, 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2 , 0.01% gelatin, 0.2 mM each dNTP, 1 μM primer, and 1 unit Taq polymerase (Promega, Madison, USA). The cycle parameters for PCR amplification were programmed for an initial denaturation at 95℃ for 4 min, followed by 35 cycles at 95℃ for 30 s, annealing at different temperatures (specific to each gene/locus) for 45 s, extension at 72℃ for 45 s, and final extension at 72℃ for 7 min. Annealing temperatures were set to 55℃ for ITS, 60℃ for GAPDH, 58℃ for ACT, and 62℃ for TUB2.
Sequence alignments for the isolates in the present study and other Colletotrichum spp. [8] were conducted by MUSCLE [9] and refined with MEGA version 7 software [10], if necessary. Maximum likelihood analysis of concatenated alignments was performed using a general time-reversible model with 1,000 bootstrap replicates by MEGA version 7 software [10]. Colletotrichum bonienese (CBS 123755) was used as the outgroup taxon. Phylogenetic analysis confirmed the morphological identification, placing the isolates in the same group as reported C. gloeosporioides strains (CFCC 56190 and CBS 112999) (Fig. 3). The sequence data of the isolates for G3PDH, ACT, TUB2, and ITS were deposited in GenBank under accession numbers PQ252373–PQ252374, PQ252371–PQ252372, PQ252375–PQ252376, and PQ248257–PQ248258, respectively.
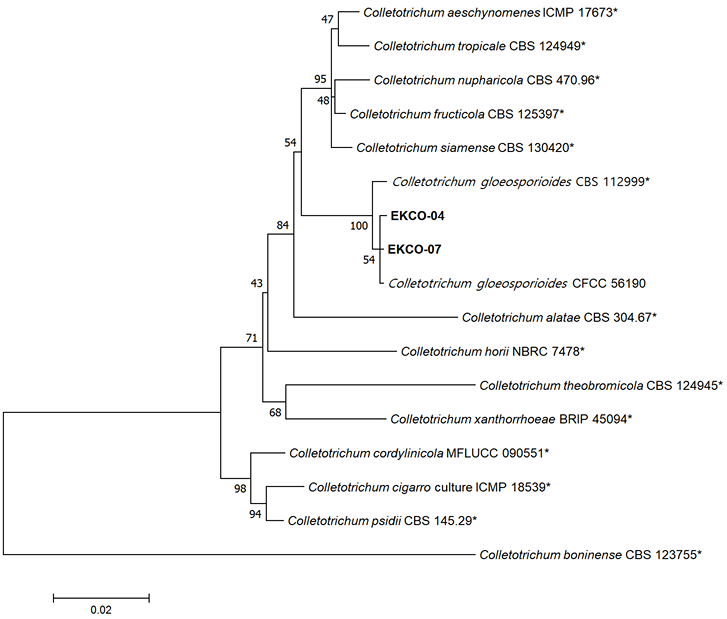
Fig. 3. Phylogenetic tree based on glyceraldehyde-3-phosphate dehydrogenase, actin, internal transcribed spacers and intervening 5.8S nrDNA, and β-tubulin gene regions of the two isolates (EKCO-04 and EKCO-07) from Korean epimedium plants and reference strains of Colletotrichum species. Sequence data for reference strains were retrieved from the National Center for Biotechnology Information (NCBI) GenBank database. The phylogenetic tree was generated using the maximum likelihood method with a general time-reversible model. Bootstrap support values are shown at the nodes. The scale bar represents the number of nucleotide substitutions per site. Asterisk (*): reference strains.
Colletotrichum spp. cause anthracnose in various plants [2,3]. Colletotrichum gloeosporioides is recognized as a species complex comprising multiple species [8]. In this study, the Colletotrichum sp. isolates causing anthracnose on Korean epimedium were identified as C. gloeosporioides based on their morphological characteristics and phylogenetic analysis. This fungus has also been reported to cause anthracnose in various plants in Korea [11]. However, no previous reports exist regarding anthracnose caused by this fungus in Korean epimedium. To the best of our knowledge, this is the first report of C. gloeosporioides causing anthracnose in Korean epimedium.
The authors declare no conflicts of interest.
This study was supported by research grants (PJ01450701 and RS-2024-00348961) from the Rural Development Administration, Korea.
1. Plants of the World Online. Epimedium koreanum Nakai [Internet]. Kew: Royal Botanic Garden; 2024 [cited 2024 August 30]. Available from https://powo.science. kew.org/.
2. Arx JA von. A revision of the fungi classified as Gloeosporium. Bibliotheca Mycologica 1970;24;1-203.
3. Sutton BC. 1992. The genus Glomerella and its anamorph Colletotrichum. In: Bailey JA and Jeger MJ, editors. Colletotrichum: Biology, Pathology and Control. Wallingford: CAB International; 1992. p. 1-26.
4. White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Inns MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315-22. [DOI]
5. Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999;91:553-6. [DOI]
6. Guerber JC, Liu B, Correll JC, Johnston PR. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 2003;95:872-95. [DOI]
7. Woudenberg JHC, Aveskamp MM, de Gruyter J, Spiers AG, Crous PW. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 2009;22:56-62. [DOI]
8. Weir BS, Johnston PR, Damm U. The Colletotrichum gloeosporioides species complex. Stud Mycol 2012;73:115-80. [DOI]
9. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004;32:1792-7. [DOI]
10. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870-4. [DOI]
11. List of Plant Diseases in Korea. [Internet]. Seoul: Korean Society of Plant Pathology; 2024. [cited 2024 August 30]. Available from http://genebank.rda.go.kr/kplant disease.do.