Sun-Mi Lee1, Hyeok Park2, and Ahn-Heum Eom1*
1Department of Biology Education, Korea National University of Education, Cheongju 28173, Korea
2Highland Agriculture Research Institute, National Institute of Crop Science, Pyeongchang 25342, Korea
*Correspondence to eomah@knue.ac.kr
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 293-299.
https://doi.org/10.4489/kjm.520408
Received on October 31, 2024, Revised on December 06, 2024, Accepted on December 06, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Orchid mycorrhizal fungi (OMF) and endophytic fungi isolated from the roots of orchid species Calanthe discolor and Cephalanthera longibracteata were inoculated onto C. discolor seedlings to examine the effects of symbiotic fungi on orchid growth. Seedlings were inoculated with nine fungal strains representing eight species and cultured for 140 days. Root and leaf growth, as well as fresh weight, were measured. The OMF species Ceratobasidium sp. and three endophytic fungal species Diaporthe hongkongensis, Pezicula ericae, and Phialocephala fortinii significantly increased seedling growth. These results indicate the potential value of orchid-symbiotic fungi to aid in the conservation and restoration of endangered and rare orchids.
Calanthe discolor, Endophytic fungi, Orchid mycorrhizal fungi, Plant growth promotion, Symbiosis
Orchidaceae plants are highly vulnerable to extinction due their sensitivity to climate change and reliance on specific pollinators [1]. Orchid restoration has become a critical research priority because their populations are rapidly declining, to the extent that eight of the 11 endangered plant species designated by the Ministry of Environment in Korea are orchids [2]. Orchids of the Calanthe genus are generally distributed in tropical and subtropical regions, and six species have been found in Korea, particularly in its southern regions and on Jeju Island [3]. Calanthe orchids have aesthetic value and potential medicinal applications as producers of anti-inflammatory and antibiotic agents [4]. However, their cultivation without specialized facilities remains challenging, and habitat destruction combined with climate change has led to their classification as endangered species in Korea [5].
Plants belonging to the Orchidaceae family establish symbiotic relationships with orchid mycorrhizal fungi (OMF) and endophytic fungi [6], which enhance nutrient uptake and influence seed germination and growth [7]. In Korea, while considerable studies have been conducted to identify and characterize species diversity of OMF and endophytic fungi [8–12], studies on the effects of symbiotic fungi on orchid growth are limited.
We aimed to inoculate sterile C. discolor seedlings with symbiotic fungi isolated from to roots of the species and determine their effects on plant growth. The objectives were to elucidate the roles of symbiotic fungi in orchid plant growth, select useful symbiotic fungal resources for enhancing orchid growth, and utilize them to propagate and restore rare native orchids in Korea.
Axenic seedlings of C. discolor Lindl. were obtained in the protocorm state from the Shinangun Agricultural Technology Center and cultured until roots formation. Environments for 150 seedlings were prepared by laying sterilized pumice stones (Youngpoong Industry, Korea) in plastic pots (9.5 cm diameter). The seedlings were then transplanted.
Nine strains belonging to eight fungal species, previous isolated from surface-sterilized orchid roots [13], were used as inocula (Table 1). Eight strains were isolated from C. discolor and one from Cephalanthera longibracteata (Table 1). The fungal strains were identified by analyzing the sequences of the internal transcribed spacer (ITS) region of rDNA using the primers ITS1 and ITS4 [14]. Sequences were deposited in GenBank. The fungal species included two OMF (Ceratobasidium sp. and Tulasnella sp.), and six non-mycorrhizal endophytic fungi (Diaporthe hongkongensis, Dactylonectria pauciseptata, Ilyonectria cyclaminicola, Leptodontidiup orchidicola, Pezicula ericae, and Phialocephala fortinii). Two strains of I. cyclaminicola were used to determine whether different strains of the same species isolated from different hosts would exert varying effects on plant growth. The fungal strains were cultured on potato dextrose agar (PDA) medium for two weeks at 25℃. Inocula were prepared by harvesting discs from established cultures using a 0.95 cm diameter cork borTer.
Table 1. Fungal strains used for orchid inoculation in this study.
| Strain | Species | GenBank Accession No. | Collection Sites | Hosts |
|---|---|---|---|---|
| Lo | Leptodontidium orchidicola | PQ517212 | Taean, Chungcheongnam-do | Calanthe discolor |
| Tul | Tulasnella sp. | PQ520467 | Jeju, Jeju-do | |
| eIc | Ilyonectria cyclaminicola | PQ517213 | ||
| Pf | Phialocephala fortinii | PQ520468 | Seogwipo, Jeju-do | |
| Pe | Pezicula ericae | PQ517243 | ||
| Dp | Dactylonectria pauciseptata | PQ517225 | ||
| Cb | Ceratobasidium sp. | PQ517224 | ||
| Dh | Diaporthe hongkongensis | PQ517227 | ||
| Ic | Ilyonectria cyclaminicola | PQ517223 | Yesan, Chungcheongnam-do | Cephalanthera longibracteata |
Pumice stones (Youngpoong Industry, Korea) were sterilized twice at 121℃ for 20 min and used as the culture medium. Sterilized pumice stones (50 g) were placed in plastic pots (9.5 cm diameter) and disinfected with 1% sodium hypochlorite (NaOCl). Healthy seedlings were selected and planted in pots, and the remaining space was filled with 25 g of pumice stones. Five inoculum discs (diameter 0.95 cm ×height 0.4 cm) per seedling were attached to the roots, ensuring contact between the mycelium and roots. Nine treatment groups and one control group were established, each with 15 replicates. Discs soaked in sterile PDA medium were placed in the control group to account for potential effects of PDA medium.
After inoculation, seedlings were cultured for 20 weeks at 23 ± 1℃ and 60 ± 10% humidity with a 12-h photoperiod. Fertilizer (Hyponex 6.5-6-19, Hyponex Japan, Osaka, Japan; diluted 1000-fold with water) was applied weekly. Pot locations were randomized periodically to eliminate location effects. Watering was discontinued three days before harvest. After 20 weeks, seedlings were carefully harvested.
Shoot and root fresh weights (FW) for all seedlings were measured before inoculation and after harvest. The number of roots and the length of the longest root were measured at both time points. The number and length of leaves were recorded every two weeks. Leaf length, root number, and FW data were analyzed using one-way ANOVA, followed by post-hoc comparisons conducted using Fisher’s least significant difference test in SPSS (version 22.0, IBM Corp., Chicago, IL, USA).
The inoculations of D. hongkongensis produced the highest number of new leaves, but no statistically significant differences were observed among the treatment groups (p > 0.05; data not shown). A significant post-inoculation difference (p < 0.05) in average leaf length was observed among the treatment groups. The average leaf length of seedlings treated with D. hongkongensis was significantly greater than those treated with L. orchidicola. However, no significant difference was noted compared to the control group (Fig. 1). A significant difference in the number of new roots was also observed among treatment groups (p < 0.05), with a significantly increase in seedlings treated with D. hongkongensis compared to that in the control group (Fig. 2). No significant difference in the length of the longest root was found (p > 0.05).
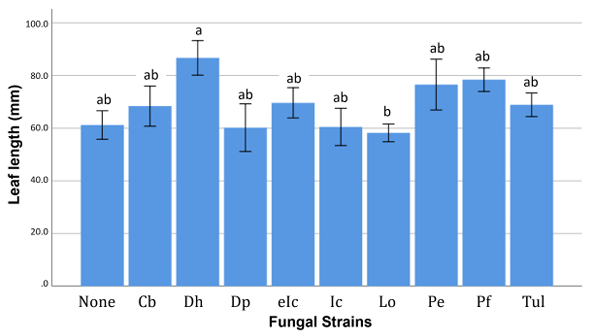
Fig. 1. Leaf length of Calanthe seedlings inoculated with different fungal strains. Each different letter (a, ab, b) indicates that p < 0.05.
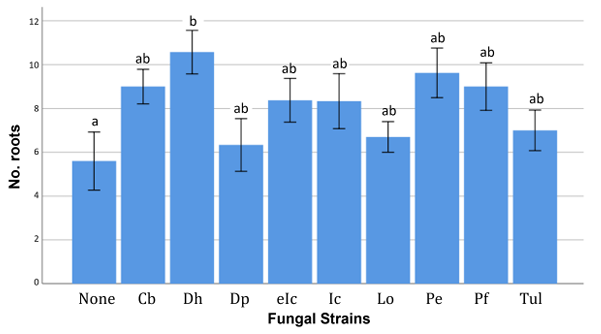
Fig. 2 Number of roots of Calanthe seedlings inoculated with different fungal strains. Different letters above each bar indicate significance at p < 0.05.
Measurements of FW of seedlings revealed significant differences among treatment groups. In aboveground tissue (shoot) FW, D. hongkongensis, P. ericae, and P. fortinii increased significantly compared to that in the control group (p < 0.05), and in belowground tissue (root) FW, only P. ericae increased significantly (p < 0.05). Total FW increased significantly in seedlings treated with P. ericae and P. fortinii (p < 0.05). FW growth rate from inoculation to harvest increased significanTtly with Ceratobasidium sp., D. hongkongensis, P. ericae, and P. fortinii treatments (Table 2, Fig. 3)
Table 2. Fresh weight (FW) of Calanthe seedlings inoculated with different fungal strains.
| Strain | Shoot (g) Mean ± SE | Root (g) Mean ± SE | Total FW (g) Mean ± SE | FW increment rate a Mean ± SE |
|---|---|---|---|---|
| None | 0.55 ± 0.11 | 0.54 ± 0.08 | 1.09 ± 0.18 | 0.01 ± 0.02 |
| Cb | 0.75 ± 0.15 | 0.56 ± 0.11 | 1.32 ± 0.25 | 0.34 ± 0.10* |
| Dh | 1.01 ± 0.14* | 0.67 ± 0.10 | 1.69 ± 0.21 | 0.41 ± 0.11* |
| Dp | 0.48 ± 0.09 | 0.42 ± 0.07 | 0.91 ± 0.15 | 0.02 ± 0.08 |
| eIc | 0.62 ± 0.09 | 0.50 ± 0.07 | 1.12 ± 0.15 | 0.12 ± 0.10 |
| Ic | 0.64 ± 0.16 | 0.51 ± 0.12 | 1.15 ± 0.27 | −0.03 ± 0.13 |
| Lo | 0.62 ± 0.10 | 0.57 ± 0.09 | 1.18 ± 0.18 | 0.16 ± 0.07 |
| Pe | 0.97 ± 0.21* | 0.97 ± 0.22* | 1.94 ± 0.42* | 0.71 ± 0.20* |
| Pf | 0.99 ± 0.14* | 0.85 ± 0.19 | 1.83 ± 0.31* | 0.58 ± 0.12* |
| Tul | 0.70 ± 0.12 | 0.67 ± 0.12 | 1.37 ± 0.24 | 0.15 ± 0.14 |
Asterisks indicate that p < 0.05.
a FW increment rate = FW of seedlings after harvest / FW of seedlings before inoculation.
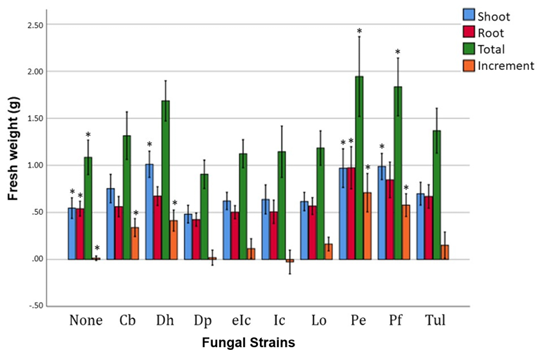
Fig. 3. Growth increment of Calanthe seedlings inoculated with different fungal strains. Asterisks indicate significance at p < 0.05.
The growth response of orchid seedlings to endophytic fungal inoculation varied depending on the strain used. Compared to the other strains, P. ericae and P. fortinii promoted an overall increase in FW in seedlings, and D. hongkongensis effectively increased the number and length of leaves, as well as the number of roots. P. ericae forms erichoid mycorrhizae in Ericaceae plants and enhances host plant resistance to water stress [15]. The results of this study suggest that P. ericae may act as an endophytic fungus in orchids to promote growth. Further research is needed to confirm its specific role and relationship with the host. P. fortinii is known to promote orchid germination and seedling growth by forming a specific endophytic symbiosis called the dark septate endophytes in orchid roots [16]. Similar results were obtained in this study. D. hongkongensis is recognized as a pathogen in Prunus spp.[17,18]. However, it promoted seedling growth in orchids, suggesting that it can function as an endophyte under certain conditions. Further studies are required to confirm its role in orchids. Seedlings treated with the OMF strain Ceratobasidium sp. showed a significant increase in FW compared to the control group, similar to the results of previous studies showing its positive effect on orchid seedling growth [19]. This result demonstrates that Ceratobasidium sp. is an OMF effective in promoting C. aristulifera seedling growth. In contrast, no significant differences were observed with Tulasnella sp. inoculation. OMF can transfer nutrients for absorption by orchids only when pelotons are decomposed. Orchid growth effects may thus depend on the relative rates of peloton formation and decomposition within the root cells [20]. In the present study, the absence of a significant promotional effect due to symbiosis may have been influenced by factors such as host specificity, experimental duration, or other variables. Further studies are required to explore this hypothesis.
To investigate potential strain-specific effects, we compared two strains of I. cyclaminicola, Ic and eIc, isolated from different hosts. No significant differences were observed between these two strains, and neither strain showed a significant growth effect compared with the control group for all values. The Ic group showed a decrease in FW compared to the control group, but the difference was not statistically significant (p > 0.05). I. cyclaminicola has often been isolated as an endophytic fungus from domestic orchids [21], and a previous study reported that it promotes growth and development of the medicinal plant Epimedium koreanum [22].
Although the effects varied depending on the growth index measured, when the overall results were summarized, Ceratobasidium sp., D. hongkongensis, P. ericae, and P. fortinii contributed to increased biomass compared to the other species and were considered to be effective species for orchid growth. These results suggest that symbiotic fungi can be used for the restoration and conservation of endangered orchid habitats.
We confirmed the growth response of orchid seedlings following inoculation with symbiotic fungi, and showed that the growth effect varied greatly depending on the fungal species. We also confirmed that both OMF and endophytic fungi could effectively promote orchid seedling growth. These results suggest that the application of symbiotic orchid fungi is an effective tool for the conservation and restoration of endangered and rare orchids.
The authors declare that there are no conflicts of interest.
None.
1. Swarts ND, Dixon KW. Terrestrial orchid conservation in the age of extinction. Ann Bot 2009;104:543-56. [DOI]
2. Ministry of Environment. Red data book of Republic of Korea. Vol. 5. Vascular plants. 2nd ed. Incheon: Nation Institute of Biological Resource; 2021.
3. Kim SH, Lee JS, Lee GJ, Kim JS, Ha BK, Kim DS, Kim JB, Kang SY. Analyses of genetic diversity and relationships in four Calanthe taxa native to Korea using AFLP markers. Hortic Environ Biotechnol 2013;54:148-55. [DOI]
4. Yoshikawa M, Murakami T, Kishi A, Sakurama T, Matsuda H, Nomura M, Matsuda H, Kubo M. Novel indole S, O-bisdesmoside, calanthoside, the precursor glycoside of tryptanthrin, indirubin, and isatin, with increasing skin blood flow promoting effects, from two Calanthe species (Orchidaceae). Chem Pharm Bull 1998;46:886-8. [DOI]
5. Lee JS, Choi BH. Distributions and red data of wild orchids in the Korean peninsula. Korean J Pl Taxon 2006;36:335-60. [DOI]
6. Rasmussen HN. Recent developments in the study of orchid mycorrhiza. Plant Soil 2002;244:149-63. [DOI]
7. Dearnaley JDW. Further advances in orchid mycorrhizal research. Mycorrhiza 2007;17:47586. [DOI]
8. Lee SS, Riew HK, Paek KY. Isolations of orchid Mycorrhizal fungi from the Korean Native orchid plants. Kor J Mycol 1997;25:101-10.
9. Sim MY, Youm JY, Chung JM, Lee BC, Koo CD, Eom AH. Characteristic of orchid mycorrhizal fungi from roots of Cypripedium japonicum and C. macranthum. Kor J Mycol 2010;38:1-4. [DOI]
10. Lee BH, Han HK, Eom AH. Identification of orchid mycorrhizal fungi isolated from terrestrial orchids in Mt. Hambaek, Korea. Kor J Mycol 2015;43:129-32.
11. Lee BH, Kim DY, Park H, Eom AH. Notes on endophytic fungi isolated from roots of Oreorchis patens in Korea. Kor J Mycol 2016;44:184-7. [DOI]
12. Lee BH, Kwon WJ, Kim JY, Park JS, Eom AH. Differences among endophytic fungal communities isolated from the roots of Cephalanthera longibracteata collected from different sites in Korea. Mycobiology 2017;45:312-7. [DOI]
13. Lee SM. Effects of symbiotic fungi isolated from the roots of Korean native orchids on the seedling growth of Calanthe discolor. Cheongju: Korea National University of Education; 2020.
14. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8. [DOI]
15. Wei X, Zhang W, Zulfiqar F, Zhang C, Chen J. Ericoid mycorrhizal fungi as biostimulants for improving propagation and production of ericaceous plants. Front Plant Sci 2022;13:1027390. [DOI]
16. Zimmerman E, Peterson RL. Effect of a dark septate fungal endophyte on seed germination and protocorm development in a terrestrial orchid. Symbiosis 2007;43:45-52.
17. Zhang Z, Zhang ZB, Huang YT, Wang FX, Hu WH, Dai LY, Zhong J, Liu Y, Zhu JZ. First report of Diaporthe hongkongensis causing fruit rot on peach (Prunus persica) in China. Plant Dis 2021;105:2017. [DOI]
18. Chen P, Abeywickrama PD, Ji S, Zhou Y, Li X, Zhang W, Yan J. Molecular identification and pathogenicity of Diaporthe eres and D. hongkongensis (Diaporthales, Ascomycota) associated with cherry trunk diseases in China. Microorganisms 2023;11:2400. [DOI]
19. Durán-López ME, Caroca-Cáceres R, Jahreis K, Narváez-Vera M, Ansaloni R, Cazar ME. The micorryzal fungi Ceratobasidium sp. and Sebacina vermifera promote seed germination and seedling development of the terrestrial orchid Epidendrum secundum Jacq. S Afr J Bot 2019;125:54-61. [DOI]
20. Dearnaley J, Perotto S, Selosse MA. Structure and development of orchid mycorrhizas. In: Martin E, editor. Molecular mycorrhizal symbiosis. Hoboken: Wiley; 2016. [DOI]
21. Lee BH, Eom AH. Diversity of endophytic fungi associated with roots of Calanthe discolor and Cephalanthera longibracteata in Korea. Kor J Mycol 2018;46:427-35.
22. Chen J, Hu X, Bai Y, Liu H, Zhuang X, Guo J, Xiao J. Diaporthe cotoneastri and Ilyonectria cyclaminicola endophytes promote the growth, development, and accumulation of active components of Epimedium koreanum Nakai host plants in field experiments. Ann Microbiol 2023;73:29. [DOI]