Jin-Sil Choi1, Seong-Keun Lim1, Gwang-Jae Lim1, Diane Avalos-Ruiz2, Seung-Yeol Lee1,3*, and HeeYoung Jung1,3
1Department of Plant Medicine, Kyungpook National University, Daegu 41566, Korea
2Centro de Excelencia Microbiano, El Jocotillo 01065, Guatemala
3Institute of Plant Medicine, Kyungpook National University, Daegu 41566, Korea
*Correspondence to leesy1123@knu.ac.kr
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 311-323.
https://doi.org/10.4489/kjm.520410
Received on September 23, 2024, Revised on December 11, 2024, Accepted on December 12, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Twenty-two strains of zygomycetous fungi were isolated from soil and selected based on their cultural characteristics. The strains were identified through similarity searches and a phylogenetic analysis of their internal transcribed spacer regions and 28S rDNA large subunit sequences. Cultural and morphological characteristics, including colony color and the sizes of sporangia, sporangiospores, and chlamydospores, were used to confirm the identity of KNUF-GWL1 as Linnemannia amoeboidea, a species not previously reported in Korea. Additionally, the twenty-two strains were evaluated for ice nucleation activity (INA) using a tube freezing test at −5℃. Among the isolated strains, five exhibited INA: KNUF-GWL1, KNUF-CNM1, KNUF-GBM2, KNUF-GBM3, and KNUF-GBM4. To assess the optimum growth temperature and the effect of growth temperature on the INA of INA-positive fungi, strains L. amoeboidea KNUF-GWL1 and Mortierella alpina KNUF-GBM4 were tested. Both strains exhibited their most vigorous growth at 25℃, with the number of ice nuclei increasing at lower incubation temperatures. To our knowledge, this study represents the first evaluation of INA in zygomycetous fungi isolated from soil and the first report of INA in L. amoeboidea.
Ice nucleation activity, Linnemannia amoeboidea, Phylogenetic analysis, Zygomycetous fungi
Zygomycetous fungi, such as Mucoromycota and Mortierellomycota, comprise about 1% of true fungi [1,2]. Most zygomycetous fungi function as saprotrophs; however, some species act as parasites on animals, plants, and other fungi, while others form mutualistic associations with plants, such as mycorrhizal relationships [2]. These fungi are classified based on their reproductive structures, such as zygospores and sporangia, and characterized by their rapidly growing mycelia [1,3]. Among zygomycetous fungi, Mucoromycota is the most extensively reported phylum, with 436 known species [3,4]. This phylum consists of various genera, including Cunninghamella and Umbelopsis, primarily isolated from soil [4]. Currently, 15 species of Cunninghamella have been documented, classified based on morphological characteristics including sporangiola shape, vesicle shape, and colony color [3,5]. The genus Umbelopsis comprise 24 species, with species of this genus characterized by slow growth, the formation of a velvety layer, and sporangia and sporangiospores of various shapes [3,6]. Similar to Mucoromycota, species within Mortierellomycota are commonly isolated from plant roots, decaying plant material, and soil [1]. In 2020, the reclassification of the family Mortierellaceae including Mortierellomycota resulted in the incorporation of seven newly described monophyletic genera, such as Linnemannia, Benniella and Podila [7]. All species of Podila produce sporangiospores, exhibiting a range of morphologies, from globose to fusoid [7]. Species belonging in the genus Podila are usually found in agricultural and forest soil, dung, and compost [8]. The genus Linnemannia comprises over 26 species, most of which are associated with decaying plant matter or the plant rhizosphere [7,9]. The closely related genus Mortierella includes 173 species, characterized by pale white or white colonies that typically form a rosette pattern [1,10].
While pure water can maintain its liquid state down to −38℃, the presence of various impurities can initiate a phenomenon known as ice nucleation, thereby elevating the freezing point [11,12]. Particles that induce ice nucleation are termed ice nucleating particles (INPs) and include minerals, dust, pollen, and biological materials [13,14]. Biological INPs are widespread in the atmosphere, typically demonstrating ice nucleation activity (INA) even at temperatures above −15℃ [13]. The most well-known biological INPs are bacteria, which are capable of inducing the freezing of supercooled water at temperatures up to −1℃ [15]. Bacterial INA is attributed to a protein anchored to the outer membrane of bacterial cells [16]. Although INA has also been observed with fungi as a biological INP at relatively high temperatures, such as −1℃ [17], the mechanisms underlying fungal INA and the characteristics of fungal INPs remain poorly understood. INA-positive particles are detected in the atmosphere and are shown to influence cloud formation, potentially leading to precipitation [18]. Additionally, ice nucleation active bacteria and fungi has been implicated in inducing frost damage to plants [19]. According to previous studies, INA has been reported in microorganisms such as Pseudomonas syringae, Fusarium acuminatum, Puccinia species, and Isaria farinosa [20–23]. The few known INA fungi are primarily classified within the phyla Ascomycota and Basidiomycota [24], highlighting the lack of the distribution of fungal INA in zygomycetous fungi.
The purpose of this study was to investigate the INA of zygomycetous fungi isolated from soil, and in this report, we identify a previously unreported species within the genus Linnemannia that exhibits INA in Korea. Additionally, the optimum growth temperature and impact of growth temperatures on INA were evaluated for fungal strains showing INA.
Diverse forest soil samples were collected in Chungbuk, Chungnam, Gangwon, Gyeongbuk, and Gyeongnam provinces in Korea. The samples were collected from a depth of 10–15 cm using sterile spatulas, transferred into sterile polythene bags, and stored at 4℃. For each soil sample, 1 g was suspended in 10 mL of sterile double-distilled water (DDW) and vortexed until dissolved [25]. The suspension was serially diluted and then plated on potato dextrose agar (PDA; Difco, Detroit, MI, USA) plates. After incubating the PDA plates for 3 days at 25℃, single colonies were transferred to new plates, followed by incubation at the same temperature. Based on descriptions from previous studies, twenty-two fungal strains of zygomycetous fungi were identified and selected for further evaluation of INA [5–8].
Total genomic DNA was extracted from a total of twenty-two fungal strains using the HiGeneTM Genomic DNA Prep Kit (BioFACT, Daejeon, Korea) according to its manufacturer’s protocol. The internal transcribed spacer (ITS) regions and 28S rDNA large subunit (LSU) were amplified using the primer pairs ITS1F/ITS4 and LROR/LR5, respectively [26–29]. The amplified PCR products were purified with ExoSAP-IT (Thermo Fisher Scientific, Waltham, MA, USA) and sequenced by Solgent Co., Ltd. (Daejeon, Korea). A Basic Local Alignment Search Tool (BLAST) search of the National Center for Biotechnology Information (NCBI) GenBank database was used to identify closely related sequences and assess their similarity. The acquired sequences of five strains KNUF-GWL1, KNUF-CNM1, KNUF-GBM2, KNUFGBM3, and KNUF-GBM4 were phylogenetically compared with related sequences retrieved from the NCBI GenBank database (Table 1) based on the concatenated ITS and LSU sequences. Sequences were aligned and uninformative regions were deleted from the alignments. A phylogenetic tree was constructed using the neighbor-joining (NJ) method with the Kimura 2-parameter model [30] in MEGA11 software with bootstrap values based on 1,000 resamples [31].
Table 1. The strains and GenBank accession numbers of the sequences used for the phylogenetic analysis in this study
| Species | Strain numbers | Accession numbers | |
|---|---|---|---|
| ITS | LSU | ||
| Linnemannia acrotona | CBS 386.71T | JX975921 | HQ667405 |
| Linnemannia amoeboidea | CBS 889.72T | JX976073 | HQ667422 |
| Linnemannia amoeboidea | KNUF-GWL1 | PQ060449 | PQ060477 |
| Linnemannia camargensis | CBS 221.58T | JX975949 | HQ667408 |
| Mortierella alpina | CBS 396.91 | JX975994 | KC018375 |
| Mortierella alpina | CBS 608.70 | MH859872 | KC018438 |
| Mortierella alpina | KNUF-CNM1 | PQ062228 | PQ062240 |
| Mortierella alpina | KNUF-GBM2 | PQ062234 | PQ062241 |
| Mortierella alpina | KNUF-GBM3 | PQ060448 | PQ060476 |
| Mortierella alpina | KNUF-GBM4 | PQ062235 | PQ062242 |
| Mortierella angusta | CBS 293.61T | JX976061 | HQ667358 |
| Mortierella antarctica | CBS 609.70T | JX975907 | HQ667423 |
| Mortierella indohii | CBS 720.71T | JX975856 | HQ667377 |
| Mortierella kuhlmanii | CBS 157.71T | JX975846 | HQ667372 |
| Mortierella microzygospora | CBS 880.97T | JX976027 | HQ667394 |
| Mortierella parazychae | CBS 868.71T | JX975985 | HQ667362 |
| Mortierella polygonia | CBS 685.71T | JX975900 | HQ667378 |
| Umbelopsis isabellina | CBS 127635 | MH864646 | MH876082 |
ITS: internal transcribed spacer regions; LSU: 28S rDNA large subunit.
T Type strain. Strains isolated in this study are indicated in bold.
The strain KNUF-GWL1 was selected for analysis of its cultural and morphological characteristics, following the previous study [7]. The characteristics were studied by incubating the strain on different media, including PDA, malt extract agar (MEA; Difco, Detroit, MI, USA), and potato carrot agar (PCA; HiMedia, Bombay, India), for 7 days at 25℃. Different features, such as colony size, color, and shape, were documented. The morphological characteristics were observed using a light microscope (BX-50; Olympus, Tokyo, Japan).
Twenty-two fungal strains were incubated on PDA for 7 days at 25℃. Subsequently, 0.01 g of mycelium from each isolated strain was respectively transferred into sterile 1.7 mL tubes containing 1 mL of DDW, with five replicates per strain, followed by thorough homogenization. To evaluate INA, the tubes containing the suspensions were placed in a refrigerated circulator bath (JEIO Tech Co., Daejeon, Korea) for 20 min at −5℃. Strains were considered INA-positive if all five tubes froze within the given time. Fusarium tricinctum KNUF-21-F29 was used as a positive control, and F. solani KNUF-21-F27 was used as a negative control based on their INA in a previous study [32].
Two strains exhibiting INA, KNUF-GWL1 and KNUF-GBM4, were chosen, and 4 mm diameter mycelial plugs for each strain were prepared using a cork borer. These plugs were each cultured on PDA at 10, 25, and 30℃ for 10 days, with three replicates for each strain and temperature. The radial growth was measured along two perpendicular axes intersecting at the center of the plug.
The above two INA-positive strains were cultured for 30 days at different temperatures and then 0.1 grams of their mycelia was collected and suspended in 10 mL of UltraPure™ Distilled Water (Invitrogen, Carlsbad, CA, USA). The suspension was vortexed for 1 min and then filtered through a 5 μm pore diameter syringe filter (Acrodisc, PES, Pall, Germany). The filtrates were serially diluted up to 10−8 with ultrapure distilled water. Aluminum foil was formed into a tray shape, and 30 droplets of 10 μL were dispensed for each sample. The aluminum foil trays with droplets were floated on an ethanol-ice mixture in a styrofoam container [32]. To test INA, the droplets were incubated for 1 min at temperatures of −3, −5, − 7, −9, −11, and −13℃. Ultrapure distilled water served as the negative control, and the number of frozen droplets was documented following each temperature treatment. The number of ice nuclei (IN) per gram of mycelium was calculated using the modified version of Vali’s formula presented by Fröhlich-Nowoisky et al. [24], and 95% confidence intervals were calculated. The number of IN per gram of mycelium was averaged over all dilutions, and the mean number of IN per gram of mycelium was calculated for all three replicates.
The partial sequences of the ITS regions and the LSU were obtained for the twenty-two isolated zygomycetous fungi. According to the BLAST results, twenty-two strains were identified, with nine classified under the genus Cunninghamella, one under Linnemannia, four under Mortierella, one under Podila, and seven under Umbelopsis (Table 2). For a detailed analysis of the unreported species in Korea, sequences containing 554 and 919 bp, corresponding to the ITS regions and LSU gene sequences, respectively, were obtained for the strain KNUF-GWL1. The BLAST results of the ITS regions and LSU gene sequences showed 99.65% and 100% similarity, respectively, with L amoeboidea (CBS 889.72T). Based on the neighbor-joining (NJ) method of the phylogenetic tree (combining the ITS regions and LSU sequences), fungal isolate KNUF-GWL1 clustered together with a previously identified L. amoeboidea strain (CBS 889.72T) and KNUF-CNM1, KNUF-GBM2, KNUF-GBM3, and KNUF-GBM4 clustered together with the M. alpina strains (CBS 396.91 and CBS 608.70) (Fig. 1).
Table 2. List of the strains isolated in this study, with their sample collecting location and species identification
| Strain | Location | Species |
|---|---|---|
| KNUF-CBC1 | Chungcheongbuk-do, Cheongju-si, Seowon-gu | Cunninghamella elegans |
| KNUF-CBC2 | Chungcheongbuk-do, Jecheon-si, Bongyang-eup | Cunninghamella elegans |
| KNUF-CNC3 | Chungcheongnam-do, Asan-si, Eumbong-myeon | Cunninghamella elegans |
| KNUF-GBC4 | Gyeongsangbuk-do, Yeongju-si, Hamang-dong | Cunninghamella elegans |
| KNUF-GBC5 | Gyeongsangbuk-do, Cheongsong-gun, Bunam-myeon | Cunninghamella elegans |
| KNUF-GBC6 | Gyeongsangbuk-do, Mungyeong-si, Dongno-myeon | Cunninghamella elegans |
| KNUF-GBC7 | Gyeongsangbuk-do, Yeongju-si, Hamang-dong | Cunninghamella elegans |
| KNUF-GBC8 | Gyeongsangbuk-do, Yeongju-si, Hamang-dong | Cunninghamella elegans |
| KNUF-GWC9 | Gangwon-do, Yeongwol-gun, Jungdong-myeon | Cunninghamella elegans |
| KNUF-GWL1 | Gangwon-do, Yeongwol-gun, Jungdong-myeon | Linnemannia amoeboidea (= M. amoeboidea) |
| KNUF-CNM1 | Chungcheongnam-do, Geumsan-gun, Jinsan-myeon | Mortierella alpina |
| KNUF-GBM2 | Gyeongsangbuk-do, Sangju-si, Hwabuk-myeon | Mortierella alpina |
| KNUF-GBM3 | Gyeongsangbuk-do, Yeongju-si, Hamang-dong | Mortierella alpina |
| KNUF-GBM4 | Gyeongsangbuk-do, Yeongju-si, Sunheung-myeon | Mortierella alpina |
| KNUF-GNP1 | Gyeongsangnam-do, Yangsan-si, Sangbuk-myeon | Podila horticola (= M. horticola) |
| KNUF-GNU1 | Gyeongsangnam-do, Yangsan-si, Sangbuk-myeon | Umbelopsis sp. |
| KNUF-GNU2 | Gyeongsangnam-do, Yangsan-si, Sangbuk-myeon | Umbelopsis sp. |
| KNUF-GBU3 | Gyeongsangbuk-do, Andong-si, Gilan-myeon | Umbelopsis sp. |
| KNUF-GBU4 | Gyeongsangbuk-do, Mungyeong-si, Dongno-myeon | Umbelopsis sp. |
| KNUF-GBU5 | Gyeongsangbuk-do, Uiseong-gun, Geumseong-myeon | Umbelopsis sp. |
| KNUF-GBU6 | Gyeongsangbuk-do, Yeongju-si, Hamang-dong | Umbelopsis sp. |
| KNUF-GBU7 | Gyeongsangbuk-do, Yeongju-si, Sunheung-myeon | Umbelopsis sp. |
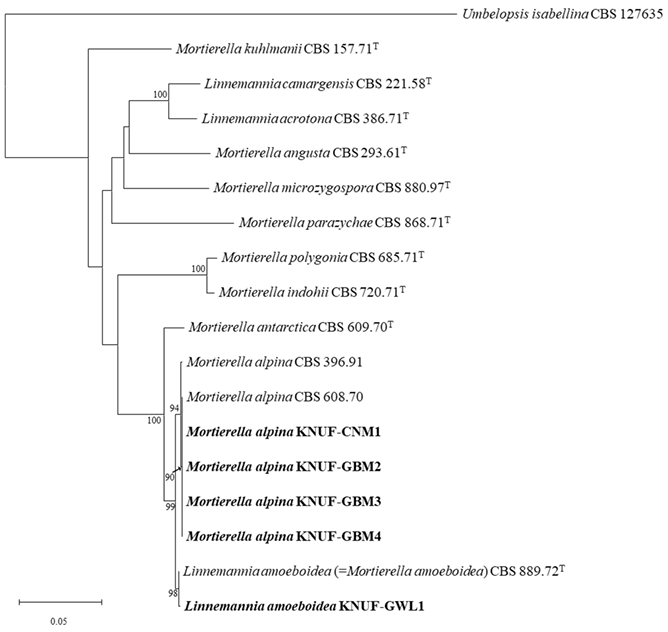
Fig. 1. Neighbor-joining phylogenetic tree based on the concatenated sequences of the internal transcribed spacer regions and 28S rDNA large subunit sequences showing the phylogenetic positions of the isolated strains (bold) among members of the family Mortierellaceae and the close relationship of KNUF-GWL1 with Linnemannia amoeboidea. The bootstrap values are based on 1,000 resamples, and values below 70 are not shown. Umbelopsis isabellina CBS 127635 was used as an outgroup. The scale bar represents 0.05 substitutions per nucleotide position.
The colonies of strain KNUF-GWL1 reached a diameter of 36–40 mm on PDA and 32–33 mm on MEA after the seven-day incubation at 25℃. On PCA medium, a relatively fast growth rate was observed, with colonies measuring 48–54 mm across. Colonies growing on PDA were densely lobed at the margin and white with abundant aerial hyphae at the center (Fig. 2A). On MEA, colonies were white, circular, and flat, showing sparse aerial mycelium (Fig. 2B). The colonies on PCA were irregularly shaped, flat, and cottony, with the same white color observed on other media (Fig. 2C). Sporulation was poor on PDA, MEA, and PCA. Sporangia were hyaline, sub-globose to oval, smooth, and multi-spored, measuring 7.1–18.9 × 7.016.2 μm (av. 14.0 × 11.9 μm, n=10) (Fig. 2D –F). Sporangiophores, arising from aerial and surface hypha, were hyaline, erect, and 56.4–124.8 × 2.4–6.6 μm (av. 89.6 × 3.7 μm, n=14). Upon the maturation of the sporangium, a collarette at the tip of the sporangiophore was observed after the dehiscence of the peridium (Fig. 2G and H). Sporangiospores were ellipsoidal and smooth-walled, measuring 3.0–4.5 × 2.3–3.3μm (av. 3.7 × 2.8 μm, n=30) (Fig. 2I). Chlamydospores were light brown, globose to irregular amoebalike, and 8.8–16.8 × 8.1–16.2 µm (av. 11.7 × 11.1 μm, n=10) (Fig. 2J). Size of sporangiophores and sporangiospores for KNUF-GWL1 were smaller than previously reported for Linnemannia amoeboidea CBS 889.72T (Table 3) [33]. However, the cultural characteristics and sporangia size were similar to those of L. amoeboidea CBS 889.72T and were differ from those of the closely related species Mortierella alpina [34]. Therefore, the strain KNUF-GWL1 was identified as L. amoeboidea which is unreported species in Korea and deposited as a metabolically inactive stock culture (KCTC 56969) in the Korean Collection for Type Cultures (KCTC). The genus Linnemannia is primarily distributed in the rhizosphere, with some species reported for their powerful chitinolytic activity [8,10]. Saprophytic fungi that decompose polymers like chitin and cellulose make plant residues more accessible to decomposition by other microorganisms, contributing to the formation of organic soils [10]. Additionally, it has been revealed that L. hyalina can promote plant growth [8], suggesting that the genus Linnemannia may have a significant role in agricultural ecosystems.
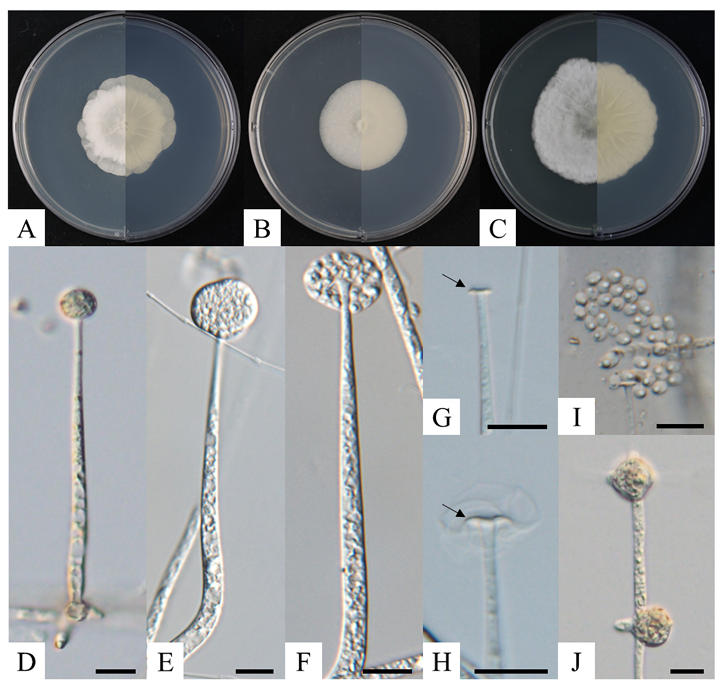
Fig. 2. Cultural and morphological characteristics of Linnemannia amoeboidea KNUF-GWL1 after 7 days at 25℃. Colony images, including front (left half) and reverse (right half) views, are shown on potato dextrose agar (A), malt extract agar (B), and potato carrot agar (C). Images showing the sporangium and sporangiophore (D–F), collarette at the tip of the sporangiophore (black arrows; G, H), sporangiospores (I), and chlamydospores (J) (scale bars in D–I = 10 μm).
Table 3. Morphological characteristics of KNUF-GWL1 and a previously described Linnemannia amoeboidea strain
| Characteristics | Linnemannia amoeboidea KNUF-GWL1a | Linnemannia amoeboidea CBS 889.72Tb | |
|---|---|---|---|
| Colony | Diameter (mm) | PDA: 36–40 MEA: 32–33 PCA: 48–54 | 5–7 mm daily radial increment in MEA |
| Color | White | N/A | |
| Shape | PDA: lobed at the margin, abundant aerial mycelium at the center MEA: circular, flat, sparse aerial mycelium PCA: irregular, flat, cottony | N/A | |
| Sporangia | Size (μm) | 7.1–18.9 × 7.0–16.2 | 10.0–15.0 |
| Sporangiophore | Size (μm) | 56.4–124.8 × 2.4–6.6 | 150.0–260.0 |
| Sporangiospore | Size (μm) | 3.0–4.5 × 2.3–3.3 | 6.0–11.0(–13.0) × 3.5–5.0 |
| Shape | Ellipsoidal, smooth-walled | Ellipsoidal, sometimes curved, smooth-walled | |
| Chlamydospore | Size (μm) | 8.8–16.8 × 8.1–16.2 | 30.0–45.0, but smaller chlamydospores also abundant |
| Color | Light brown | Light brown | |
| Shape | Globose to irregular amoeba-like | Irregular amoeba-like |
PDA: potato dextrose agar; MEA: malt extract agar; PCA: potato carrot agar; N/A: not available in reference.
a Fungal strain isolated in this study; b Source of description [33]. TType strain.
Among the twenty-two strains, one strain of Linnemannia amoeboidea KNUF-GWL1 and four strains Mortierella alpina KNUF-CNM1, KNUF-GBM2, KNUF-GBM3, and KNUF-GBM4 exhibited high INA. In this study, we modified the amount of mycelium described by Fröhlich-Nowoisky et al. [24], to perform the tube-freezing assay by extracting 0.01 g of mycelium that was then suspended in 1 mL of DDW. All f ive tubes for each strain froze, similar to the positive control, Fusarium tricinctum KNUF-21-F29 (Fig. 3). The remaining seventeen strains exhibited negative INA, with none of the five tubes freezing. M. alpina has previously been reported as ice nucleation active fungus, representing the first known case of INA in zygomycetous fungi [24]. In this study, we determined that L. amoeboidea is also one of the zygomycetous fungi showed INA. This result suggests that the INA observed in L. amoeboidea may draw in moisture and water in dry soils, potentially contributing to processes such as germination, as has been proposed for M. alpina [24].
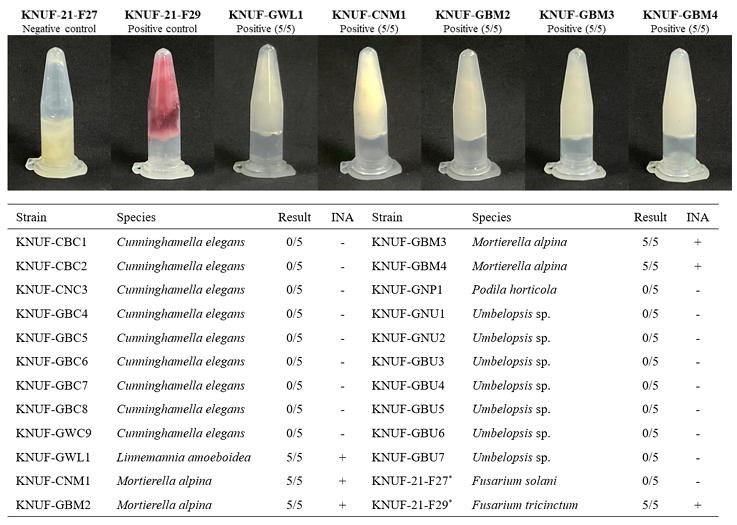
Fig. 3. Results of the tube freezing test conducted at −5℃ for 20 minutes on 22 isolated strains. Images include the five isolated strains showing ice nucleation activity (INA) and the negative (Fusarium solani KNUF-21-F27) and positive (F. tricinctum KNUF-21-F29) control strains based on their INA in a previous study [32]. *: INA-negative control (KNUF-21-F27) and INA-positive control (KNUF-21-F29) used in the study.
To compare mycelial growth at different temperatures among INA-positive strains, Linnemannia amoeboidea KNUF-GWL1 and Mortierella alpina KNUF-GBM4 were selected. Four strains of M. alpina exhibited identical cultural and morphological characteristics, leading to selection of KNUF-GBM4 for further analysis. The mycelial growth diameters of KNUF-GWL1 and KNUF-GBM4 were measured 10 days after incubation at 10, 25, and 30℃ (Fig. 4). When cultured at 10℃, the mycelial growth diameters of KNUF-GWL1 and KNUF-GBM4 were 29.85 and 24.18 mm, respectively, indicating reduced growth compared to cultivation at higher temperatures. Both KNUF-GWL1 and KNUF-GBM4 exhibited their most vigorous growth when cultured at 25℃, with mycelial growth diameters of 52.34 and 56.50 mm, respectively. At 30℃, KNUF-GBM4 displayed a decreased mycelial growth diameter, 27.04 mm, compared to its growth at 25℃, while KNUF-GWL1 exhibited a mycelial growth diameter of 52.12 mm, comparable to its growth at 25℃. Compared to both strains, mycelial growth at 10 and 25℃ showed no significant differences; however, at 30℃, the diameters of the strains differed by more than 25 mm. These results suggest that the growth of strain KNUF-GWL1 is less affected by high temperatures compared to KNUF-GBM4.
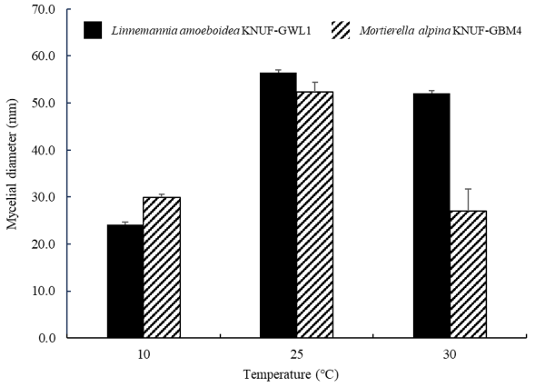
Fig. 4. Mycelial growth of Linnemannia amoeboidea KNUF-GWL1 and Mortierella alpina KNUFGBM4 after 10 days of incubation at different temperatures. Mean growth diameter of KNUF-GWL1 and KNUF-GBM4 from three replicates are given. Error bars represent the standard deviation.
To investigate the impact of growth temperature on fungal INA, Linnemannia amoeboidea KNUFGWL1 and Mortierella alpina KNUF-GBM4 were grown at 10, 25, and 30℃ for 30 days. When the two strains were incubated at 10℃, the number of IN/g of mycelium at −13℃ was similarly highest, measuring 4 × 1011 IN/g (Fig. 5). In contrast, strains grown at 25℃ exhibited significant differences, with KNUFGWL1 showing 2 × 1010 IN/g and KNUF-GBM4 showing 8 × 109 IN/g, respectively. The two strains incubated at 30℃ showed the lowest number of IN per gram of mycelium at −13℃, with approximately 106 IN/g for both strains, compared to the different incubation temperatures. Additionally, the number of IN per gram of mycelium for both strains grown at 30℃ represented significant differences at treatment temperature of −5 and −7℃, with L. amoeboidea KNUF-GWL1 exhibiting higher INA than M. alpina KNUF-GBM4 at same temperatures. Both L. amoeboidea KNUF-GWL1 and M. alpina KNUF-GBM4 exhibited higher INA when grown at lower temperatures, a phenomenon similar to that found in Fusarium avenaceum [35]. As suggested in previous studies, INA genes may be expressed at higher levels under lower temperatures, leading to the production of more INPs [35]. However, research on the INA genes and INPs associated with L. amoeboidea remains limited. In addition, mycelial growth for both KNUF-GWL1 and KNUF-GBM4 was reduced at 10℃, while the number of IN per gram of mycelium increased at the same incubation temperature. This result suggests that there may be no direct correlation between vigorous growth and high INA. Notably, recent climate changes have led to extreme weather events such as cold waves, ice nucleation-active fungi have been reported to play a significant role in causing frost damage to plants [36,37]. Although zygomycetous fungi especially are mainly known to be as saprophyte or soil inhabitant [38,39], the further study of INA-positive M. alpina and newly reported L. amoeboidea might be important to understand their ecological characters including effect on plants. Furthermore, researches on the INPs of M. alpina has identified various characteristics, including size, heat stability, and enzymatic and chemical stability [24], while there have been no studies on L. amoeboidea. In this reason, further researches are needed to characterize the INPs of L. amoeboidea for understanding its INA.
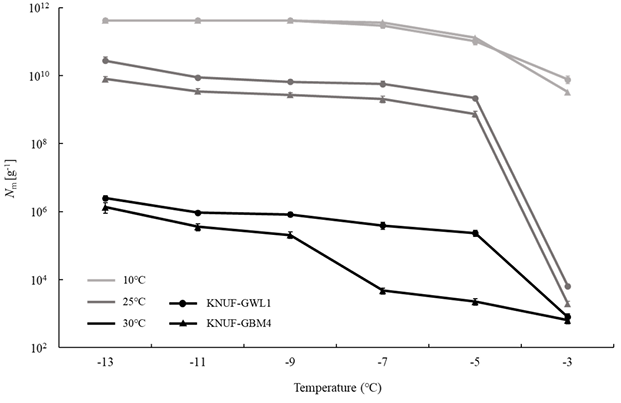
Fig. 5. Average number of ice nuclei (IN) g-1 of mycelium for Linnemannia amoeboidea KNUF-GWL1 and Mortierella alpina KNUF-GBM4 at different incubation temperatures. These strains incubated on PDA for 30 days at each temperature. Error bars indicate the 95% confidence intervals.
The authors declare no conflict of interest.
None.
1. Goh J, Oh Y, Mun HY. First reports of unrecorded Mortierellomycetes and Umbelopsidomycetes fungi from freshwater ecosystems in Korea. Kor J Mycol 2023;51:15565.
2. Krings M, Taylor TN, Dotzler N. Fossil evidence of the zygomycetous fungi. Persoonia 2013;30:1-10. [DOI]
3. Zhao H, Nie Y, Zong TK, Wang K, Lv ML, Cui YJ, Tohtirjap A, Chen JJ, Zhao CL, Wu F, et al. Species diversity, updated classification and divergence times of the phylum Mucoromycota. Fungal Divers 2023;123:49-157. [DOI]
4. Voigt K, James TY, Kirk PM, Santiago ALCMA, Waldman B, Griffith GW, Fu M, Radek R, Strassert JFH, Wurzbacher C, et al. Early-diverging fungal phyla: taxonomy, species concept, ecology, distribution, anthropogenic impact, and novel phylogenetic proposals. Fungal Divers 2021;109:59-98. [DOI]
5. Nguyen TTT, Choi YJ, Lee HB. Isolation and characterization of three unrecorded zygomycete fungi in Korea: Cunninghamella bertholletiae, Cunninghamella echinulata, and Cunninghamella elegans. Mycobiology 2017;45:318-26. [DOI]
6. Wang YN, Liu XY, Zheng RY. Four new species records of Umbelopsis (Mucoromycotina) from China. J Mycol 2013;2013:970216. [DOI]
7. Vandepol N, Liber J, Desirò A, Na H, Kennedy M, Barry K, Grigoriev IV, Miller AN, O’Donnell K, Stajich JE, et al. Resolving the Mortierellaceae phylogeny through synthesis of multi-gene phylogenetics and phylogenomics. Fungal Divers 2020;104:267-89. [DOI]
8. Telagathoti A, Probst M, Mandolini E, Peintner U. Mortierellaceae from subalpine and alpine habitats: new species of Entomortierella, Linnemannia, Mortierella, Podila and Tyroliella gen. nov. Stud Mycol 2022;103:25-58. [DOI]
9. Telagathoti A, Probst M, Peintner U. Habitat, snow-cover and soil pH, affect the distribution and diversity of Mortierellaceae species and their associations to bacteria. Front Microbiol 2021;12:669784. [DOI]
10. Ozimek E, Hanaka A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021;11:7. [DOI]
11. Failor KC, Liu H, Llontop MEM, LeBlanc S, Eckshtain-Levi N, Sharma P, Reed A, Yang S, Tian L, Lefevre CT, et al. Ice nucleation in a Gram-positive bacterium isolated from precipitation depends on a polyketide synthase and non-ribosomal peptide synthetase. ISME J 2022;16:890-7. [DOI]
12. Lagzian M, Latifi AM, Bassami MR, Mirzaei M. An ice nucleation protein from Fusarium acuminatum: cloning, expression, biochemical characterization and computational modeling. Biotechnol Lett 2014;36:2043-51. [DOI]
13. Huang S, Hu W, Chen J, Wu Z, Zhang D, Fu P. Overview of biological ice nucleating particles in the atmosphere. Environ Int 2021;146:106197. [DOI]
14. Margaritis A, Bassi AS. Principles and biotechnological applications of bacterial ice nucleation. Crit Rev Biotechnol 1991;11:277-95. [DOI]
15. Morris CE, Georgakopoulos DG, Sands DC. Ice nucleation active bacteria and their potential role in precipitation. J Phys IV France 2004;121:87-103. [DOI]
16. Lukas M, Schwidetzky R, Eufemio RJ, Bonn M, Meister K. Toward understanding bacterial ice nucleation. J Phys Chem B 2022;126:1861-7. [DOI]
17. Richard C, Martin JG, Pouleur S. Ice nucleation activity identified in some phytopathogenic Fusarium species. Phytoprotection 1996;77:83-92. [DOI]
18. Morris CE, Conen F, Alex Huffman J, Phillips V, Pöschl U, Sands DC. Bioprecipitation: a feedback cycle linking Earth history, ecosystem dynamics and land use through biological ice nucleators in the atmosphere. Glob Chang Biol 2014;20:341-51. [DOI]
19. Schwidetzky R, de Almeida Ribeiro I, Bothen N, Backes AT, DeVries AL, Bonn M, FröhlichNowoisky J, Molinero V, Meister K. Functional aggregation of cell-free proteins enables fungal ice nucleation. Proc Natl Acad Sci U S A 2023;120:e2303243120. [DOI]
20. Maki LR, Galyan EL, Chang-Chien MM, Caldwell DR. Ice nucleation induced by Pseudomonas syringae. Appl Microbiol 1974;28:456-9. [DOI]
21. Pouleur S, Richard C, Martin JG, Antoun H. Ice nucleation activity in Fusarium acuminatum and Fusarium avenaceum. Appl Environ Microbiol 1992;58:2960-4. [DOI]
22. Morris CE, Sands DC, Glaux C, Samsatly J, Asaad S, Moukahel AR, Gonçalves FLT, Bigg EK. Urediospores of rust fungi are ice nucleation active at > −10℃ and harbor ice nucleation active bacteria. Atmos Chem Phys 2013;13:4223-33. [DOI]
23. Huffman JA, Prenni AJ, DeMott PJ, Pöhlker C, Mason RH, Robinson NH, Fröhlich-Nowoisky J, Tobo Y, Després VR, Garcia E, et al. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos Chem Phys 2013;13:6151-64. [DOI]
24. Fröhlich-Nowoisky J, Hill TCJ, Pummer BG, Yordanova P, Franc GD, Pöschl U. Ice nucleation activity in the widespread soil fungus Mortierella alpina. Biogeosciences 2015;12:1057-71. [DOI]
25. Das K, Lee SY, Jung HY. Molecular and morphological characterization of two novel species collected from soil in Korea. Mycobiology 2020;48:9-19. [DOI]
26. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8. [DOI]
27. White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press, Inc.; 1990. p. 315-22. [DOI]
28. Rehner SA, Samuels GJ. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res 1994;98:625-34. [DOI]
29. Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 1990;172:4238-46. [DOI]
30. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 1980;16:111-20. [DOI]
31. Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 2021;38:3022-7. [DOI]
32. Avalos-Ruiz D, Ten LN, Kim CK, Lee SY, Jung HY. Isolation and identification of ice nucleation active Fusarium strains from rapid apple declined trees in Korea. Plant Pathol J 2022;38:403-9. [DOI]
33. Gams W. Some new or noteworthy species of Mortierella. Persoonia 1976;9:111-40.
34. Yadav DR, Kim SW, Babu AG, Adhikari M, Kim C, Lee HB, Lee YS. First report of Mortierella alpina (Mortierellaceae, Zygomycota) isolated from crop field soil in Korea. Mycobiology 2014;42:401-4. [DOI]
35. Yang S, Rojas M, Coleman JJ, Vinatzer BA. Identification of candidate ice nucleation activity (INA) genes in Fusarium avenaceum by combining phenotypic characterization with comparative genomics and transcriptomics. J Fungi 2022;8:958. [DOI]
36. Jeon MJ, Cho Y. An analysis of a winter-time temperature change and an extreme cold waves frequency in Korea. J Climate Change Res 2015;6:87-94. [DOI]
37. Lee SY, Peter KA, Das K, Diane AR, Jung HY. The rapid apple decline phenomenon: current status and expected associated factors in Korea. Plant Pathol J 2023;39:538-47. [DOI]
38. Lee JS, Nam B, Lee HB, Choi YJ. Molecular phylogeny and morphology reveal the underestimated diversity of Mortierella (Mortierellales) in Korea. Kor J Mycol 2018;46:37582.
39. Nguyen TTT, Lee HB. Isolation and characterization of three zygomycetous fungi in Korea: Backusella circina, Circinella muscae, and Mucor ramosissimus. Mycobiology 2018;46:317-27. [DOI]