Seung-Yoon Oh* and Yehyeon Cha
Department of Biology and Chemistry, Changwon National University, Korea
*Correspondence to syoh@changwon.ac.kr
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 331-340.
https://doi.org/10.4489/kjm.520412
Received on December 09, 2024, Revised on December 12, 2024, Accepted on December 12, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
Terrestrial isopods such as Porcellio laevis play a critical role in nutrient cycling and serve as habitats for diverse microbial communities. Although bacterial diversity in isopods has been widely studied, research on fungal diversity remains limited. In the present study, we examined the mycobiome of P. laevis to investigate sex-specific differences in the fungal composition using a metabarcoding approach. The results revealed significant differences in fungal community composition between sexes, despite no variation in alpha diversity. Females were enriched with fungi, such as Aspergillus and Fusarium, which are commonly found in leaf litter, whereas males showed higher proportions of Stereum and Penicillium, which are associated with wood decay. These findings are consistent with those of previous studies on other arthropods, thus emphasizing the influence of host sex on microbial communities. Our study highlights the ecological dynamics that shape fungal communities in P. laevis and highlights the need for further research to explore the functional roles of sexspecific taxa and their implications for host ecology.
Arthropods, Diversity, Fungi, Isopod, Woodlice
Terrestrial isopods, commonly referred to as woodlice, are detritivores that play a vital role in soil nutrient cycling [1]. These organisms contribute significantly to the breakdown of organic matter, decomposition of plant material, and enhancement of nutrient availability to other organisms in ecosystems [2]. In addition to their ecological contributions, terrestrial isopods harbor diverse microbial communities, including bacteria and fungi [3–5], which can influence their health, digestion, and ability to adapt to various environmental conditions. Although studies on the bacterial microbiome of terrestrial isopods have revealed diverse bacterial interactions that shape their ecology and physiology [6], existing research on the fungal community or mycobiome remains limited. This highlights the need for deeper exploration of the diversity and functional roles of fungi associated with terrestrial isopods. Fungi serve diverse roles within the isopod mycobiome by forming mutualistic relationships that assist in digestion and act as opportunistic pathogens [7,8]. Moreover, fungi can act as a direct food source for isopods, making diet a crucial factor in shaping the mycobiome composition [9,10]. Their reliance on fungi as part of their diet indicates that the fungal communities present in their environment can directly influence the composition of their internal mycobiomes.
Previous studies on Armadillidium spp. have shown that different host species harbor distinct mycobiome communities, thereby highlighting the influence of host-specific factors on fungal diversity [5]. However, the potential variation within a single species, particularly between males and females, remains largely unknown. Host sex can influence microbial communities due to differences in hormonal profiles, immune function, behavior, and ecological niches, making it an important factor to explore in microbiome research [11]. In arthropods, sex-specific differences in microbial communities have been observed in various taxa and are often linked to hormonal influences that affect immune responses or the physiological environment of the host [12]. Understanding these sex-based differences can provide valuable insights into the ecological and evolutionary dynamics of host-microbe interactions.
Terrestrial isopods are commonly found in the forests, gardens, and urban environments of South Korea [13]. A total of 19 species of terrestrial isopods, classified into eight families and 17 genera, have been reported in South Korea [13]. Among these isopods, P. laevis is a cosmopolitan species distributed worldwide across Europe, Asia, and the Americas [14]. This species thrives in a wide range of environments, from temperate forests to human-modified landscapes, and is frequently found in areas rich in decaying organic matter such as leaf litter and wood debris [15]. It plays a crucial role in nutrient recycling by breaking down organic material, making it a vital component of soil ecosystems [16]. Despite their ecological significance, studies on the fungal communities associated with P. laevis, particularly regarding the differences between male and female hosts, remain limited. In the present study, we examined the mycobiome of P. laevis with a focus on uncovering sex-specific differences. We aimed to characterize the fungal community structure and diversity of this species and evaluate how host sex influences the mycobiome using a metabarcoding approach targeting the nuclear ribosomal internal transcribed spacer (ITS) region. Our findings enhance the understanding of how host traits shape microbial communities and shed light on the ecological roles of fungi in terrestrial isopods.
P. laevis individuals were collected from an urban park in Gwacheon, South Korea in October 2022. We collected eight individuals for the experiment, two males and two females, from each of the two locations. From site 1 (St1), two female samples (PF1 and PF2) and two male samples (PM1 and PM2) were collected from site 2 (St2), and two female samples (PF3 and PF4) and two male samples (PM3 and PM4) were also collected. Male and female individuals were identified based on their morphological characteristics, and samples were stored at −20℃ until further processing.
The collected samples were surface sterilized with 70% ethanol, rinsed with sterile distilled water, and dried. For DNA extraction, the samples were homogenized using a Taco™Prep Bead Beater (GeneReach, Taichung, Taiwan), and genomic DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). For the metabarcoding analysis, the fungal ITS1 region was amplified using the Illumina adaptor-attached primers ITS1Fngs and ITS2ngs [17,18]. Polymerase chain reactions (PCR) amplification was performed under the following conditions: 95℃ for 5 min; 25 cycles of 94℃ for 30 s, 55℃ for 30 s, 72℃ for 40 s; and a final extension at 72℃ for 5 min. Under the same conditions, the number of cycles was reduced to 15 for the second PCR amplification to attach the identifier barcodes and sequencing adaptors. The results were confirmed using 1% agarose gel electrophoresis and the PCR products were purified using an Expin PCR SV kit (GeneAll, Seoul, South Korea). Each sample was amplified in triplicate, combined into a single sample, and quantified using a Multiskan SkyHigh Microplate Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of DNA from all samples were pooled and sequenced using the Illumina MiSeq platform at Macrogen (Seoul, South Korea). To quantify the fungal ITS load present in isopod DNA samples, quantitative PCR (qPCR) was performed using the Luna Universal qPCR Master Mix (New England Biolabs, Frankfurt, Germany) and a QuantStudio 6 Pro RealTime PCR system (ThermoFisher Scientific, Waltham, USA) at the Gyeongnam Bio and Anti-aging Core Facility Center (Changwon National University, South Korea). qPCR was conducted using the same primer sets and amplification conditions as the standard PCR for 40 cycles. Relative quantification values were calculated based on the average Ct value of female samples, as described in a previous study [19].
Raw sequences were processed, and bioinformatic analyses were conducted following previously established methods [5]. Briefly, ASVs were generated using the QIIME2 and DADA2 pipelines [20,21], followed by clustering into operational taxonomic units (OTUs) at 97% similarity using VSEARCH [22]. Taxonomic assignment was performed using a naïve Bayesian classifier based on the UNITE v.10 database [23,24]. Non-fungal sequences were eliminated and the remaining sequences were standardized to the minimum value (29,000 sequences) for subsequent analyses. Further analysis and visualization were conducted using the phyloseq and vegan packages in R [25,26]. Alpha diversity indices including richness (Chao1), diversity (Shannon), and evenness (Pielou) were calculated. For beta diversity, Bray-Curtis-based non-metric multidimensional scaling (NMDS) analysis was conducted first, followed by permutational multivariate analysis of variance (PERMANOVA) tests using the adonis function. Comparative analyses of alpha diversity and relative abundance were conducted using t-tests after verifying statistical assumptions. To identify host sex-specific fungal lineages, a Linear discriminant analysis Effect Size (LEfSe) analysis was performed using the microeco package with a significance threshold of 0.05, and the top 30 taxa with an LDA score of 3 [27].
MiSeq sequencing generated a total of 627,015 reads (50,434–108,257 reads per sample), of which 472,486 reads (38,478–79,725 reads) remained. After removing non-fungal sequences, 362,285 reads (29,297–69,854 reads) and 84 OTUs (11–67 OTUs per sample) were identified. Good’s coverage ranged 0.999–1.000, indicating a sufficient sequencing depth. A comparison of fungal alpha diversity indices (richness, diversity, and evenness) and relative ITS copy numbers between the host sexes revealed no statistically significant differences across all values (Fig. 1).
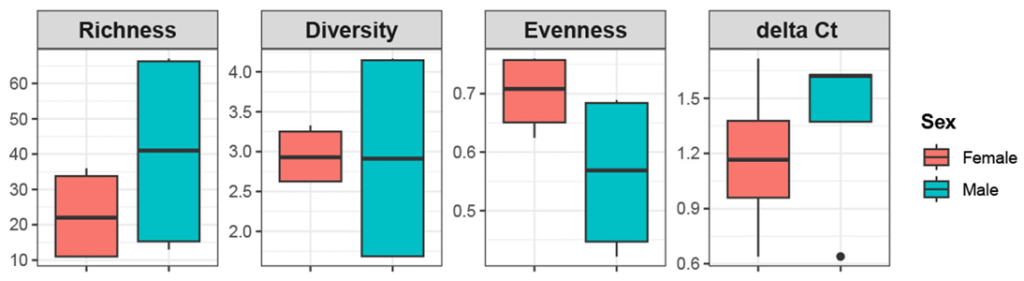
Fig. 1. Alpha diversity indices and internal transcribed spacer (ITS) copy loads for male and female P. laevis. Boxplot showing the richness (Chao1), diversity (Shannon), evenness (Pielou), and delta Ct (ITS copy loads) of the fungal community in male and female P. laevis. No significant differences were observed between host sex.
NMDS analysis based on Bray-Curtis dissimilarity showed clear clustering of fungal communities according to host sex (Fig. 2). PERMANOVA confirmed that the differences in community composition between males and females were statistically significant (R2 = 45.9%, P = 0.004). In addition, mycobiomes were significantly different between the sampling sites (R2 = 22.6%, P = 0.027). Across all samples of P. laevis, fungal taxa were classified into three phyla, nine classes, 21 orders, 41 families, and 45 genera. The distribution of fungal communities in female and male hosts was compared at multiple taxonomic levels (Fig. 3). At the phylum level, Ascomycota was dominant in all samples (77.7–99.7%), and Basidiomycota was also abundant in male samples (20.5–22.3%) (Fig. 3A). At the class level, Eurotiomycetes was dominant (16.6–71.7%), but females were dominated by Sordariomycetes (50.6–68.8%), whereas males were dominated by Agaricomycetes (20.5–21.5%) and Dothideomycetes (0.0–34.6%) (Fig. 3B). At the order level, Eurotiales was dominant (16.2–69.9%). Females were dominated by Hypocreales (41.7–48.4%), whereas males were dominated by Cladosporiales (20.5–21.5%) or Russulales (0.0–34.6%) (Fig. 3C). At the family level, Aspergillaceae was consistently dominant (16.2–68.7%). In females, Nectriaceae (33.7–40.1%) was dominant, whereas males showed dominance by Cladosporiaceae (7.2–20.9%) or Stereaceae (0.0–27.5%) (Fig. 3D). Statistically significant differences were observed at the genus level for Aspergillus, Fusarium, Penicillium, and Stereum (Fig. 4). Females showed significantly higher proportions of Aspergillus (15.7–38.0%) and Fusarium (33.7–40.0%), whereas males exhibited significantly higher proportions of Penicillium (6.6–65.9%) and Stereum (7.2–20.9%). Consistent with these results, LEfSe analysis highlighted taxonomic differences between the sexes (Fig. 5). Females were enriched in Aspergillus and Fusarium within the Ascomycota, whereas males were enriched in Stereum within the Agaricomycetes (Basidiomycota) and Cyphellophora, Ophiognomonia, and Penicillium within the Ascomycota.
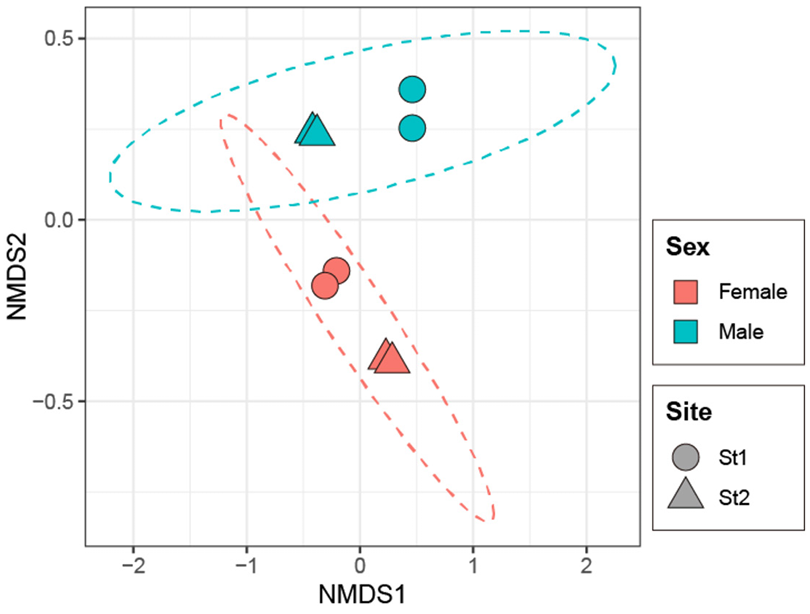
Fig. 2. Non-metric multidimensional scaling (NMDS) plot based on Bray-Curtis dissimilarity for fungal communities associated with P. laevis.
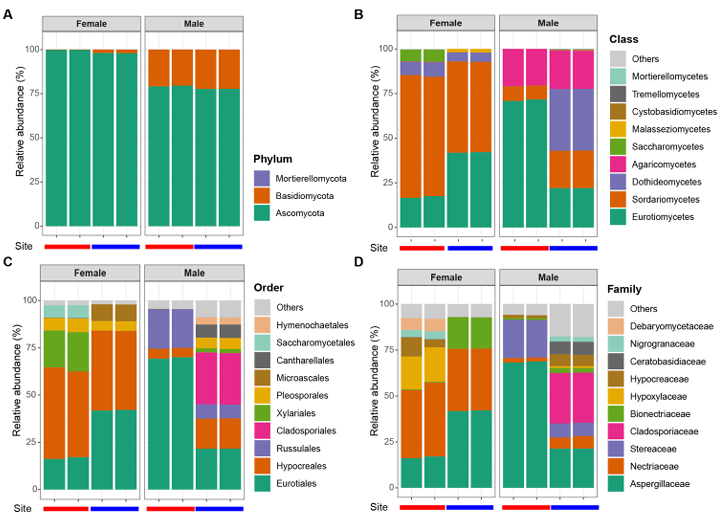
Fig. 3. Community composition of major fungal taxa in P. laevis for (A) phylum, (B) class, (C) order, and (D) family levels.
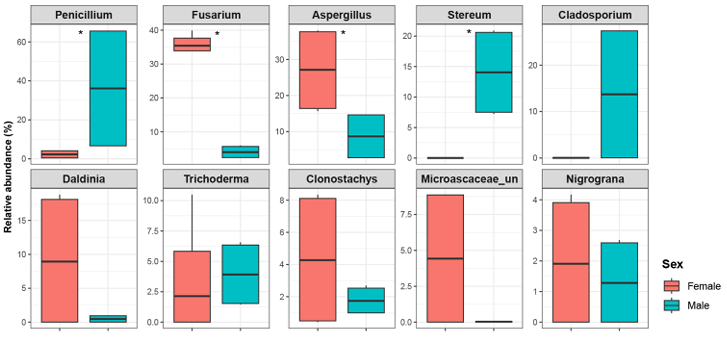
Fig. 4. Relative abundance of major fungal genera in P. laevis. Asterisks (*) denote statistically significant differences determined using the Student’s t-test (P < 0.05).
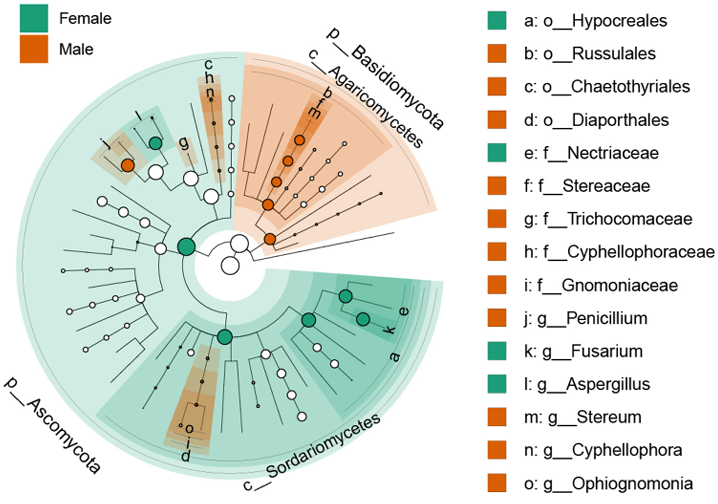
Fig. 5. Linear discriminant analysis Effect Size (LEfSe) cladogram showing the differential abundance of fungal taxa between male and female of P. laevis.
The present study revealed that the mycobiome of P. laevis was influenced by the sex of the host, showing significant differences in community composition, although no differences were observed in overall fungal diversity (alpha diversity). These results are consistent with those of previous research on other arthropods, which emphasized the role of host sex in influencing microbial communities [12]. To the best of our knowledge, this is the first study to document sex-specific differences in the mycobiome of P. laevis, providing novel insights into the factors driving fungal diversity in terrestrial isopods.
A comparison with other terrestrial isopods such as Armadillidium vulgare provides additional context for these findings [5,28]. A previous study on A. vulgare had similarly identified Aspergillus, Fusarium, and Penicillium as the dominant taxa in their fungal communities [5], indicating that these fungi are broadly adapted to colonize terrestrial isopods. However, the presence of Daldinia and Stereum dominant taxa in P. laevis highlights species-specific differences in the fungal community composition. Notably, Stereum, a member of the Stereaceae, is associated with wood decay [29], which aligns with the behavioral tendency of P. laevis to inhabit decaying wood environments [15]. Fusarium, known for its plant-pathogenic or saprotrophic properties [30], is found in leaf litter, which explains its dominance in female habitats. Fusarium is also recognized as an insect pathogen [31]. This suggests that Fusarium may have entered through incidental ingestion when consuming food, or with the intent of infecting terrestrial isopods.
The composition of the mycobiome varied significantly between sexes, in agreement with previous studies on bacterial communities in intertidal isopods (Jaera albifrons) [4]. The distinct community composition of males and females may be linked to differences in hormonal profiles [11], which can affect immune responses or provide specific niches that favor certain fungal taxa. Behavioral differences between sexes could also influence fungal exposure. Females may spend more time in environments rich in leaf litter, favoring the proliferation of fungi such as Aspergillus and Fusarium [32]. In males, the dominance of Stereum, Cyphellophora, Ophiognomonia, and Penicillium indicated a different set of ecological interactions. Cyphellophora species have been found in diverse habitats, including Antarctic regions [33]; however, their specific interactions with isopods remain underexplored. Ophiognomonia species are typically plant-associated and often specialize in specific host genera [34], which may reflect the microhabitats frequented by male isopods. The ecological strategies of the dominant taxa further elucidated their distribution. Aspergillus and Penicillium, despite their closely related [35], occupy distinct niches. Aspergillus thrives in drier, warmer conditions, whereas Penicillium prefers cooler, nutrient-rich environments [36]. These adaptations may reflect the microhabitats frequented by male and female P. laevis. Similarly, the presence of Stereum in males highlights its association with wood decay processes, emphasizing the ecological interplay between fungal communities and host behavior.
Although this study provides valuable insights, certain limitations should be acknowledged. First, the relatively small sample size and limited geographical coverage may restrict the generalizability of the findings. Expanding this study to include additional individuals and diverse habitats would improve the robustness of the results and provide a clearer understanding of the broader ecological dynamics of terrestrial isopods. Second, future research should explore the causal mechanisms underlying these patterns, such as hormonal influences on fungal colonization, immune responses, and sexual behavior differences. Lastly, experimental studies comparing the functional roles of dominant taxa such as Fusarium in nutrient cycling and Stereum in wood decomposition may also shed light on the ecological implications of these associations. Such insights would enhance our knowledge of host-microbe interactions and the broader ecological implications of sex-specific microbial communities in terrestrial isopods.
The authors declare that there are no conflicts of interest.
This research was supported by Changwon National University in 2023–2024.
1. Schmidt C. Phylogeny of the terrestrial Isopoda (Oniscidea): a review. Arthropod Syst Phylogeny 2008;66:191-226. [DOI]
2. Zimmer M. Nutrition in terrestrial isopods (Isopoda: Oniscidea): an evolutionary-ecological approach. Biol Rev 2002;77:455-93. [DOI]
3. Dittmer J, Lesobre J, Moumen B, Bouchon D. Host origin and tissue microhabitat shaping the microbiota of the terrestrial isopod Armadillidium vulgare. FEMS Microbiol Ecol 2016;92:fiw063. [DOI]
4. Wenzel MA, Douglas A, Piertney SB. Microbiome composition within a sympatric species complex of intertidal isopods (Jaera albifrons). PLoS One 2018;13:e0202212. [DOI]
5. Cha Y, Oh SY. Fungal diversity associated with Armadillidium isopods: a case study in Central Park of Gwacheon, South Korea. Diversity 2023;15:533. [DOI]
6. Bouchon D, Zimmer M, Dittmer J. The terrestrial isopod microbiome: an all-in-one toolbox for animal-microbe interactions of ecological relevance. Front Microbiol 2016;7:1472. [DOI]
7. Bahram M, Netherway T. Fungi as mediators linking organisms and ecosystems. FEMS Microbiol Rev 2022;46:fuab058. [DOI]
8. Cafaro MJ. Gut fungi of isopods: the genus Palavascia. Mycologia 2000;92:361-9. [DOI]
9. Crowther TW, Stanton DWG, Thomas SM, A’Bear AD, Hiscox J, Jones TH, Vorísková J, Baldrian P, Boddy L. Top-down control of soil fungal community composition by a globally distributed keystone consumer. Ecology 2013;94:2518-28. [DOI]
10. Póss AM, Bogdányi FT, Tóth F. Consumption of fungi-infected fallen pear leaves by the common woodlouse. Acta Phytopathol Entomol Hung 2022;57:79-91.
11. Kim YS, Unno T, Kim BY, Park MS. Sex differences in gut microbiota. World J Mens Health 2020;38:48-60. [DOI]
12. Malacrinò A. Host species identity shapes the diversity and structure of insect microbiota. Mol Ecol 2022;31:723-35. [DOI]
13. Kwon DH. Terrestrial Isopoda (Crustacea) from Korea. Korean J Zool 1993;36:133-58.
14. Schmalfuss H. World catalog of terrestrial isopods (Isopoda: Oniscidea). In: Fricke R, Merkl O, Nyffeler R, Pape T, Price M, Schawaller W, Schlüter A, Wake D, Wörz A, editors. Stuttgarter Beiträge zur Naturkunde, Serie A, Nr. 654. Stuttgart: Staatliches Museum für Naturkunde Stuttgart; 2003. p. 341.
15. Gregory SJ. 15 years on: An update to Woodlice and Waterlice in Britain and Ireland, part 1~ native and naturalised species. Bull Br Myriap Isopod Group 2024;36:20-58.
16. Arın L, Dinçsoy H. Effect of vermicompost and isopod (Porcellio laevis) fertilizers on the emergence and seedling quality of lettuce (Lactuca sativa var. capitata cv. Wismar). Int J Agric Environ Food Sci 2020;4:501-6. [DOI]
17. Tedersoo L, Anslan S, Bahram M, Põlme S, Riit T, Liiv I, Kõljalg U, Kisand V, Nilsson H, Hildebrand F, et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 2015;10:1-43. [DOI]
18. Tedersoo L, Tooming-Klunderud A, Anslan S. PacBio metabarcoding of fungi and other eukaryotes: errors, biases and perspectives. New Phytol 2018;217:1370-85. [DOI]
19. Choi H, Park JS, Hwang JA, Kim SK, Cha Y, Oh SY. Influence of biofloc technology and continuous flow systems on aquatic microbiota and water quality in Japanese eel aquaculture. Diversity 2024;16:601. [DOI]
20. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019;37:852-7. [DOI]
21. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: highresolution sample inference from Illumina amplicon data. Nat Methods 2016;13:581-3. [DOI]
22. Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016;4:e2584. [DOI]
23. Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Caporaso JG. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018;6:90. [DOI]
24. Abarenkov K, Nilsson RH, Larsson KH, Taylor AFS, May TW, Frøslev TG, Pawlowska J, Lindahl B, Põldmaa K, Truong C, et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: sequences, taxa and classifications reconsidered. Nucleic Acids Res 2024;52(D1):D791-7. [DOI]
25. McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013;8:e61217. [DOI]
26. Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. Community Ecology Package; 2020. p. 1-263.
27. Liu C, Cui Y, Li X, Yao M. microeco: an R package for data mining in microbial community ecology. FEMS Microbiol Ecol 2021;97:fiaa255. [DOI]
28. Crowther TW, Thomas SM, Maynard DS, Baldrian P, Covey K, Frey SD, van Diepen LTA, Bradford MA. Biotic interactions mediate soil microbial feedbacks to climate change. Proc Natl Acad Sci U S A 2015;112:7033-8. [DOI]
29. Schwarze FWMR. Wood decay under the microscope. Fungal Biol Rev 2007;21:133-70. [DOI]
30. Summerell BA. Resolving Fusarium: current status of the genus. Annu Rev Phytopathol 2019;57:323-39. [DOI]
31. Teetor-Barsch GH, Roberts DW. Entomogenous Fusarium species. Mycopathologia 1983;84:3-16. [DOI]
32. Mahongnao S, Sharma P, Nanda S. Characterization of fungal microbiome structure in leaf litter compost through metagenomic profiling for harnessing the bio-organic fertilizer potential. 3 Biotech 2024;14:191. [DOI]
33. Alves IMS, Gonçalves VN, Oliveira FS, Schaefer CEGR, Rosa CA, Rosa LH. The diversity, distribution, and pathogenic potential of cultivable fungi present in rocks from the South Shetlands archipelago, Maritime Antarctica. Extremophiles 2019;23:327-36. [DOI]
34. Walker DM, Castlebury LA, Rossman AY, Struwe L. Host conservatism or host specialization? Patterns of fungal diversification are influenced by host plant specificity in Ophiognomonia (Gnomoniaceae: Diaporthales). Biol J Linn Soc 2014;111:1-16. [DOI]
35. Houbraken J, Kocsubé S, Visagie CM, Yilmaz N, Wang XC, Meijer M, Kraak B, Hubka V, Bensch K, Samson RA, et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud Mycol 2020;95:5-169. [DOI]
36. Wicklow DT. Ecological adaptation and classification in Aspergillus and Penicillium. In: Samson RA, Pitt JI, editors. Advances in Penicillium and Aspergillus Systematics. Boston: Springer US; 1986. p. 255-65. [DOI]