Ju-Heon Lee1,2, Youngsoo Kim1, Jong-Taek Park1, Dong-Hyuk Lee1, and Hee-Young Jung2*
1Apple Research Center, National Institute of Horticultural & Herbal Science, Gunwi 43100, Korea
2Department of Plant Medicine, Kyungpook National University, Daegu 41566, Korea
*Correspondence to heeyoung@knu.ac.kr
Korean Journal of Mycology (Kor J Mycol) 2024 December, Volume 52, Issue 4, pages 381-389.
https://doi.org/10.4489/kjm.520416
Received on December 16, 2024, Revised on December 20, 2024, Accepted on December 20, 2024, Published on Dec 30, 2024.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
A fungus was isolated from ambrosia beetles collected using beetle traps in an apple orchard in Gunwi-gun, Daegu, South Korea. This fungal strain was termed ARI-24-A5, and was identified through morphological characterization and molecular phylogenetic analysis. After 8 d of incubation on potato dextrose agar (PDA), ARI-24-A5 exhibited gray-to-olive coloration, abundant aerial mycelia, and a colony diameter of 72.0–79.0 mm. Morphologically, the aleurioconidiophores formed monilioid chain structures, and the size of the aleurioconidia was 11.1 × 10.8 μm. For precise identification, molecular phylogenetic analysis was performed using the internal transcribed spacer (ITS) region, translation elongation factor 1-alpha (TEF1-α), small subunit of nuclear ribosomal RNA (SSU), and RNA polymerase II subunit 1 (RPB1) gene sequences. The overall analysis confirmed that ARI-24-A5 belongs to the genus Ambrosiella, which is known for its symbiotic relationship with ambrosia beetles. In the phylogenetic tree, ARI-24-A5 shared the same taxonomic position as A. catenulata and its morphological characteristics were consistent with those of this species. Therefore, ARI24-A5 was identified as A. catenulata, making this the first record of this species in South Korea.
Ambrosia beetle, Ambrosiella catenulata, Korean apple orchard, Symbiotic fungi
Ambrosia beetles (Coleoptera: Curculionidae) belong to the subfamilies Scolytinae and Platypodinae and are a highly diverse pest group, with 158 species reported in Korea [1]. These beetles cause significant damage to orchards, nurseries, and forest ecosystems, making them major pests [2]. Based on their ecological characteristics, beetles are classified into two types: bark beetles, which feed on the tree substrate itself, and ambrosia beetles, which bore tunnels into trees and cultivate ambrosia fungi within these tunnels for nutrition [3,4]. These unique ecological behaviors suggest that these pests cause long-term damage to trees through interactions with their symbiotic fungi.
Most of these beetles have evolved a wide range of symbiotic relationships with fungi. However, the degree and nature of these associations vary significantly, often exhibiting unique characteristics that are not observed in other beetle groups [5,6]. Similarly, the relationships among bark beetles, fungi, and their tree hosts are diverse. Some bark beetles require tree mortality for reproduction [7], whereas others feed on white-rot fungi found in decaying tree trunks [8], and some live in association with fungi in dry branches [9].
In Korea, Raffaelea quercus-mongolicae is the causal agent of oak wilt disease and has been reported to form a symbiotic relationship with Platypus koryoensis, with beetle invasion triggering the disease [1]. In addition, in the United States, ambrosia beetles (Anisandrus maiche), which contribute to the decline of apple trees, have been collected. Ambrosiella cleistominuta has been isolated from the galleries and adults of this beetle and was identified as a symbiotic fungus associated with A. maiche [2].
As mentioned, ambrosia beetles are associated with various fungi [5,10], and Batra [11] defined ʻprimary ambrosia fungi’ as fungi that co-evolve with the mycangia of the beetle and form dense spore layers inside the beetle tunnels, serving as a food source for the beetles. Most species of the genus Ambrosiella, associated with ambrosia beetles, have been reported to exhibit a strong relationship with these beetles [12]. In Korea, two species have been documented: A. grosmanniae KNU16-001 [13] from soil and A. roeperi ARI-24-A4 [14] from ambrosia beetles.
In the present study, ambrosia beetles that damaged apple trees in domestic orchards were collected using beetle traps. The fungi isolated from these beetles were identified based on morphological and molecular phylogenetic analyses. The results of these identifications are reported here.
Ambrosia beetles were collected using a beetle trap in an orchard at the Apple Research Center in Gunwigun, Daegu-si, Korea (36°29′68.9″N, 128°46′56.1″E). The beetle bodies were surface-sterilized with 70% ethanol and thoroughly dried for approximately 10 min. Subsequently, the insects were dissected by separating the head and thorax from the abdomen. Each segment was transferred onto potato dextrose agar (PDA; Difco, Detroit, MI, USA) plates and incubated at 25℃ for 3 d. The resulting mycelial growth was subcultured onto fresh PDA plates and incubated for an additional 8 d at the same temperature. The resulting pure culture was labeled as ARI-24-A5 and preserved in 20% glycerol at -80℃ for future use.
The isolated ARI-24-A5 was cultured on PDA medium at 25℃ for 8 d to examine its cultural and mycological characteristics. After cultivation, various features of the fungal colonies, such as diameter and color, were observed. The morphology and size of the conidia and conidiophores were examined and recorded using a light microscope (CX-43; Olympus, Japan).
For molecular analysis and to assess phylogenetic relationships, genomic DNA from strain ARI-24-A5 was extracted using the HiGene Genomic DNA Preparation Kit (Biofact, Daejeon, Korea) following the manufacturer’s guidelines. Subsequently, partial sequences of the internal transcribed spacer (ITS) regions, small subunit of nuclear ribosomal RNA (SSU), translation elongation factor 1-alpha (TEF1-α), and RNA polymerase II subunit 1 (RPB1) genes were amplified and obtained using PCR. The ITS regions were amplified using the ITS1F/ITS4 [15,16] and ITS5/LR3 primers [16]. The SSU gene was amplified using the NS-1/NS-6 primers [16,17]. The TEF1-α gene was amplified using the EFCF1.5/EFCF6 primer pair [18]. The RPB1 gene was amplified using the RPB1-Af/RPB1-Cr primer pair [12,19,20]. The PCR amplification products were verified through electrophoresis on 1% agarose gels, followed by staining with ethidium bromide. The resulting PCR products were purified using EXOSAP-IT reagent (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s guidelines. Sequencing was performed by Solgent Co. Ltd. (Daejeon, Korea). Sequence analysis was performed using SeqMan Lasergene software (DNAStar Inc., Madison, Wisconsin, USA). The ITS regions, SSU, TEF1-α, and RPB1 gene sequences were deposited in GenBank with accession numbers LC835911 (ITS), LC835913 (SSU), LC835915 (TEF1α), and LC848343 (RPB1), respectively.
The sequences of Ambrosiella spp. were retrieved from the National Center for Biotechnology Information (NCBI) database. These sequences were aligned using Clustal X 2.0, in MEGA 7 [21]. A phylogenetic tree was constructed based on concatenated nucleotide sequences of the ITS region, TEF1-α, and SSU genes. For the analysis, the nearest neighbor interchange method was employed using Kimura’s two-parameter model [22], excluding gaps. A phylogenetic tree was constructed using the maximum likelihood (ML) method [23], and reliability was assessed using bootstrap values from 1,000 replicates.
After culturing on PDA at 25℃ for 8 d, the colony diameter reached 72.3–79.2 mm. On the front side, white mycelia were observed in the center, which transitioned from olive to white toward the edges. In addition, the surface was covered with abundant aerial mycelia. The back side was generally olive-gray in color (Fig. 1A).
The aleurioconidiophores were hyaline, smooth, ovoid, and monilioid, with branching structures (Fig. 1B). Conidia and collarettes were formed at the terminal ends of the conidiophores (Figs. 1C and D). Aleurioconidia are hyaline, ranging from spherical to subglobose in shape, with an average size of 11.1 × 10.8 μm (n = 50), were located at the ends of the aleurioconidiophores. When the aleurioconidia detached from the terminal end, they typically carried one or two conidiophores (Fig. 1E). In most cases, aleurioconidia detach from multiple conidiophore cells during chain formation (Fig. 1F).
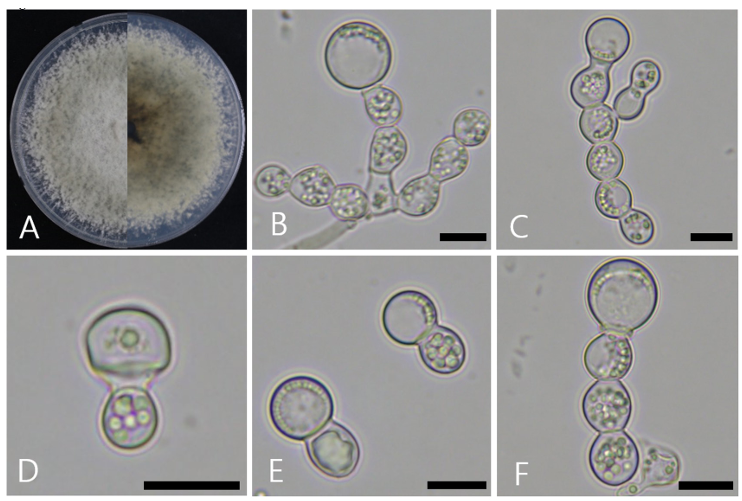
Fig. 1. Cultural and morphological characteristics of Ambrosiella catenulata. A: Front and reverse view of the colony grown on potato dextrose agar (PDA) for 8 days at 25℃. B, C: Aleurioconidiophores with terminal aleurioconidia. D: Aleurioconidiophores with collarettes. E: Aleurioconidiophores with aleurioconidia. F: Aleurioconidiophores with aleurioconidia and forms a monilioid chain. Scale bars: BF = 10 μm.
To analyze the molecular and phylogenetic relationships of ARI-24-A5, four molecular markers (ITS region, TEF1-α, SSU, and RPB1) were used. The lengths of the sequences obtained were 1,078, 1,119, 1,318, and 762 bp, respectively. A Basic Local Alignment Search Tool (BLAST) search was conducted to compare these sequences with those of other strains in the NCBI database. For the ITS region, ARI-24-A5 showed the highest similarity (100%) to Ambrosiella catenulata W186q, followed by 99.8% similarity with A. xylebori Hulcr5114 and A. cleistominuta C3843. For TEF1-α, ARI-24-A5 exhibited 100% similarity with A. catenulata W186q, 97.2% with A. xylebori CBS 110.61, and 96.3% with A. grosmanniae 1002HHS1. For SSU, ARI-24-A5 displayed 100.0% similarity with A. catenulata C3913 and 99.9% similarity with A. batrae, A. xylebori, and A. cleistominuta. Finally, RPB1 of ARI-24-A5 showed 100% similarity with three strains of A. catenulata (12B1, 12B2, and W186q), 99.1% similarity with A. xylebori JH12105, 96.5% similarity with A. grosmanniae JH12109, and 95.2% similarity with A. beaveri W204qT. This analysis indicated that ARI-24-A5 matched perfectly (100%) with A. catenulata across all four genetic markers (ITS, TEF1-α, SSU, and RPB1). To analyze the phylogenetic relationships of ARI-24-A5, a multilocus sequence analysis (MLSA) was conducted using ITS, TEF1-α, and SSU, based on the process of Mayers et al. [24], with nucleotide sequence data retrieved from the NCBI database for Ambrosiella species (Table 1). The phylogenetic tree was constructed using the ML method and confirmed that ARI-24-A5 shares the same phylogenetic position as A. catenulata and is clearly distinguishable from other species (Fig. 2). Based on comprehensive phylogenetic analyses, ARI-24-A5 was conclusively identified as identical to A. catenulata at the species level.
Table 1. The following is a list of species included in the phylogenetic analyses, along with their corresponding GenBank accession numbers
| Species | Strain | Associated ambrosia beetle | GenBank accession numbers | ||
|---|---|---|---|---|---|
| ITS | TEF1-α | SSU | |||
| Ambrosiella batrae | CBS 139735T | Anisandrus sayi | KR611322 | KT290320 | KR673881 |
| Ambrosiella catenulata | C3913 | Ambrosia beetle | MG950184 | MG944394 | MG950189 |
| Ambrosiella cleistominuta | C3843 | Anisandrus maiche | KX909940 | KX925304 | KX925309 |
| Ambrosiella grosmanniae | CBS 137359T | Xylosandrus germanus | KR611324 | KT318382 | KR673884 |
| Ambrosiella hartigii | CBS 404.82 | Anisandrus dispar | KF669873 | KT318383 | KR673885 |
| Ambrosiella nakashimae | CBS 139739T | Xylosandrus amputatus | KR611323 | KT318381 | KR673883 |
| Ambrosiella remansi | M290 | Remansus mutabilis | KX342068 | KX342072 | KX354426 |
| Ambrosiella roeperi | CBS 135864T | Xylosandrus crassiusculus | KF669871 | KT318384 | KR673886 |
| Ambrosiella xylebori | CBS 110.61T | Xylosandrus compactus | KF669874 | KT318385 | KR673887 |
| Ambrosiella catenulata | ARI-24-A5 | Ambrosia beetle | LC835911 | LC835915 | LC835913 |
ITS: internal transcribed spacer regions; TEF1-α: translation elongation factor 1-α; SSU: small subunit of nuclear ribosomal RNA.
T ex-type. The isolated strain is shown in bold.
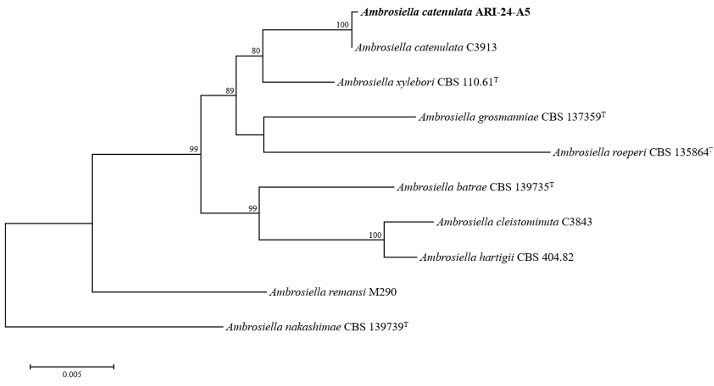
Fig. 2. Maximum-likelihood phylogenetic tree of ARI-24-A5 based on the combined sequences (ITS + TEF1-α + SSU), showing the phylogenetic position of the ARI-24-A5 strain among Ambrosiella species. Bootstrap values (based on 1,000 replications) greater than 70% are indicated at the branch points. The isolated strain is highlighted in bold. Ambrosiella nakashimae (CBS 139739T ) was used as the outgroup. Bar = 0.005 substitutions per nucleotide position. ‘T’ indicates the type strain. ITS: internal transcribed spacer regions; TEF1-α: translation elongation factor 1-α; SSU: small subunit of nuclear ribosomal RNA.
The culture and morphological characteristics of ARI-24-A5 were consistent with those of A. catenulata. A. catenulata is morphologically very similar to A. roeperi, and the ARI-24-A5 strain showed similar trends to A. roeperi in terms of the shape and size of its aleurioconidia. The size of the aleurioconidia in ARI-24-A5 was (7–)11.1(–16) × (7–)10.8(–13) μm, which was closely similar to that of the aleurioconidia in A. roeperi, which was (7–)11.0(–16) × (5–)10.0(–14) μm. In addition, both species have spherical aleurioconidia. However, when conidia dislodge, two or more conidiophore cells typically remain attached to the dislodged aleurioconidia in A. catenulata, often forming a chain-like structure, whereas in A. roeperi, typically only one cell remains. Furthermore, the sporodochia of A. catenulata were spherical, whereas those of A. roeperi appeared diffuse and cushion-like (Table 2).
Table 2. Comparison of the morphological characteristics of strain ARI-24-A5 with those of the reference species Ambrosiella catenulata and A. roeperi
| Characteristics | A. catenulataa (ARI-24-A5) | A. catenulatab | A. roeperib | |
|---|---|---|---|---|
| Colony | Color | Overall light gray color is exhibited, with olive coloration observed at the edges. Additionally, abundant aerial mycelium is formed | Olivaceous to gray, surface covered with abundant aerial mycelium | Olivaceous to gray, dark gray, to dark-brown superficial |
| Shape | Colonies on PDA attaining 72.0–79.0 mm diam after 8 days at 25℃ | Colonies on PDA attaining 52.0–59.0 mm diam after 8 days at 25℃ | Colonies on PDA attaining 60.5–70.0 mm diam after 8 days at 25℃ | |
| Aleurioconidiophores | Color | Hyaline | Hyaline | Hyaline to subhyaline |
| Shape | Smooth and ellipsoidal, forming monilioid chains, with the part attached to the spores observed as thickened septa | Smooth, ellipsoidal, monilioid, branched, sometimes the collarette appearing as thickened septa | Smooth, cylindrical, monilioid, branched, with or without a collarette on the top cell of conidiophores | |
| Aleurioconidia | Color | Hyaline | Hyaline | Hyaline |
| Shape | Thick-walled, smooth, with organelles observed, almost spherical in shape, When detaching, it mostly contains one or more conidiophore cells and forms a chain-like | Globose to subglobose, single and terminal on aleur ioconidiophores, when detaching, mostly carrying two (rarely one) or more conidiophore cells | Thick-walled, globose to subglobose, if detachable, it mostly carries a single conidiophore cell, which is cushion-like in shape | |
| Size (μm) | (7–)11.1(–16) × (7–)10.8(–13) | (8–)9–13(–16) × (7 .5–)9–12(–13) | (7–)9–13(–16) × (5–)8–12(–14) |
a Fungal strain studied in this paper; b Source of description [31].
This study aimed to analyze symbiotic fungi isolated from ambrosia beetles collected from apple trees in Korea, with a focus on the accurate identification of fungal species associated with these beetles. The fungus isolated from ambrosia beetles was identified as A. catenulata.
Species of the genus Ambrosiella Brader ex Arx & Hennebert [25] have been shown to form symbiotic relationships with ambrosia beetles, establishing dense fungal gardens known as “ambrosia”, which serve as the beetles exclusive food source [26–28]. These fungi are typically found in beetles with large and complex mesothoracic mycangia, which are specialized structures for storing and transporting fungi [12]. In contrast, beetles with smaller mycangia may host various species of the genus Raffaelea as well as other fungi. Specific species within the genus Raffaelea form symbiotic relationships with multiple beetle species [29,30]. A key morphological feature of Ambrosiella spp. is the formation of aleurioconidia, which are often attached to one or more conidiophore cells, or positioned at the tip of an independent aleurioconidiophore. Aleurioconidia may exist as single units or, rarely, as short chains and may also form a collarette at the apex of the conidiophore [31].
To distinguish Ambrosiella spp., Mayers et al. [24] used ITS regions, TEF1-α, and SSU gene sequences to differentiate species such as A. beaveri, A. ferruginea, A. hartigii, A. roeperi, and A. xylebori [12]. However, these markers are insufficient for differentiating A. nakashimae from A. beaveri. In 2017, Lin et al. [31] proposed A. catenulata as a new species based on ITS, TEF1-α, and RPB1 gene sequences. However, these markers failed to differentiate A. nakashimae from A. beaveri. This finding is consistent with the results of Mayers et al. [24] and Lin et al. [31], who reported that their phylogenetic analysis aligned with the phylogenetic tree presented by Mayers et al. [24].
For this analysis, four molecular markers (ITS, TEF1-α, SSU, and RPB1) previously applied in related studies were used to determine the molecular phylogeny of strain ARI-24-A5. A BLAST search confirmed that this strain is A. catenulata. Its closest relative was identified as A. xylebori, which showed morphological similarities to A. roeperi, although significant morphological differences were observed in the sporodochia structure. To construct the phylogenetic tree, the ITS, TEF1-α, and SSU gene sequences were analyzed following the method used by Mayers. The RPB1 sequences used in the method of Lin et al. [31] were excluded because of the lack of sequence data for most Ambrosiella specimens. Both phylogenetic trees yielded consistent results, allowing the use of a single method.
The comprehensive findings of this study revealed that ARI-24-A5 shares the same phylogenetic position as A. catenulata C3913 and is distinct from other Ambrosiella spp.. This represents the first report of A. catenulata in Korea. In addition, R. quercus-mongolicae has been reported as a symbiotic fungus of the ambrosia beetle (P. koryoensis) that damages oak trees in Korea [1]. Although reports on symbiotic fungi associated with ambrosia beetles in Korean apple orchards remain limited, in the United States, symbiotic fungi such as Ambrosiella xylebori and A. cleistominuta have been isolated from ambrosia beetles that damage apple orchards in New York [2,32]. This indicates that the ambrosia beetles that damage apple trees in Korea may also be associated with symbiotic fungi other than A. catenulata, highlighting their potential for discovering additional symbiotic fungi. Understanding these symbiotic relationships is essential to devise sustainable pest management strategies. Future research should focus on thoroughly investigating the ecological and economic impacts of A. catenulata in Korean apple orchards and exploring the influence of environmental factors on its distribution.
The authors declare that they have no potential conflicts of interest.
This study was supported by the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01718404)” funded by the Rural Development Administration, Republic of Korea.
1. Park IK. Current status of pheromone research of forest insect pests in Korea and development direction. Korean J Appl Entomol 2022;61:63-75.
2. Tobin KN, Moore ME, Lizarraga S, Petzoldt J, Reese C, Lovett B, Rivera MJ. First report of Anisandrus maiche (Coleoptera: Curculionidae: Scolytinae) infesting apple trees. Zootaxa 2024;5506:261-71. [DOI]
3. Ito M, Kajimura H. Phylogeography of bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae). J Jpn For Soc 2009;91:424-32. [DOI]
4. Masuya H, Yamaoka Y. The relationships between fungi and scolytid and platypodid beetles. J Jpn For Soc 2009;91:433-45. [DOI]
5. Harrington TC. Ecology and evolution of mycophagous bark beetles and their fungal partners. In: Vega FE, Blackwell M, editors. Insect-fungal associations: ecology and evolution. Oxford: Oxford University Press; 2005. p. 257-91. [DOI]
6. Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst 2005;36:563-95. [DOI]
7. Thatcher RC, Searcy JL, Coster JE, Hertel GD. The Southern pine beetle. Washington DC: US Department of Agriculture; 1981. USDA Forest Service Technical Bulletin 1631.
8. Li Y, Simmons DR, Bateman CC, Short DPG, Kasson MT, Rabaglia RJ, Hulcr J. New fungusinsect symbiosis: culturing, molecular, and histological methods determine saprophytic Polyporales mutualists of Ambrosiodmus ambrosia beetles. PLoS One 2015;10:e0137689. [DOI]
9. Kolarík M, Kubátová A, Hulcr J, Pazoutová S. Geosmithia fungi are highly diverse and consistent bark beetle associates: evidence from their community structure in temperate Europe. Microb Ecol 2008;55:65-80. [DOI]
10. Skelton J, Jusino MA, Li Y, Bateman C, Thai PH, Wu C, Lindner DL, Hulcr J. Detecting symbioses in complex communities: the fungal symbionts of bark and ambrosia beetles within Asian pines. Microb Ecol 2018;76:839-50. [DOI]
11. Batra LR. Ambrosia beetles and their associated fungi: research trends and techniques. Proc Indian Acad Sci 1985;94:137-48. [DOI]
12. Harrington TC, McNew D, Mayers C, Fraedrich SW, Reed SE. Ambrosiella roeperi sp. nov. is the mycangial symbiont of the granulate ambrosia beetle, Xylosandrus crassiusculus. Mycologia 2014;106:835-45. [DOI]
13. Park S, Ten L, Lee SY, Back CG, Lee JJ, Lee HB, Jung HY. New recorded species in three genera of the Sordariomycetes in Korea. Mycobiology 2017;45:64-72. [DOI]
14. Lee JH, Kim Y, Ten L, Park JT, Lee DH, Jung HY. Ambrosiella roeperi: an unreported fungus isolated from ambrosia beetles. Kor J Mycol 2024;52:247-255.
15. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8. [DOI]
16. White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315-22. [DOI]
17. Vilgalys R. Conserved primer sequences for PCR amplification and sequencing from nuclear ribosomal RNA [Internet]. Durham: Duke University; 2018 [cited 2024 Sep 10]. Available from https://sites.duke.edu/vilgalyslab/rdna_primers_for_fungi/
18. Oliveira LS, Harrington TC, Ferreira MA, Damacena MB, Al-Sadi AM, Al-Mahmooli IH, Alfenas AC. Species or genotypes? Reassessment of four recently described species of the Ceratocystis wilt pathogen, Ceratocystis fimbriata, on Mangifera indica. Phytopathol 2015;105:1229-44. [DOI]
19. Stiller JW, Hall BD. The origin of red algae: implications for plastid evolution. Proc Natl Acad Sci U S A 1997;94:4520-5. [DOI]
20. Matheny PB, Liu YJ, Ammirati JF, Hall BD. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am J Bot 2002;89:688-98. [DOI]
21. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870-4. [DOI]
22. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Mol Biol Evol 1980;16:111-20. [DOI]
23. Fitch WM. Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool 1971;20:406-16. [DOI]
24. Mayers CG, McNew DL, Harrington TC, Roeper RA, Fraedrich SW, Biedermann PH, Castrillo LA, Reed SE. Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fungal Biol 2015;119:1075-92. [DOI]
25. Brader L. Étude de la relation entre le scolyte des rameaux du caféier, Xyleborus compactus Eichh. (X. morstatti Hag.), et sa plante-hôte [dissertation]. Wageningen: Wageningen University; 1964.
26. Batra LR. Ambrosia fungi-a taxonomic revision, and nutritional studies of some species. Mycologia 1967;59:976-1017. [DOI]
27. Beaver RA. Insect-fungus relationships in the bark and ambrosia beetles. In: Wilding N, Collins NM, Hammond PM, Webber JF, editors. Insect-Fungus Interactions. London: Academic Press; 1989. p. 121-43. [DOI]
28. Kok LT. Lipids of ambrosia fungi and the life of mutualistic beetles. In: Batra LR, editor. Insect-Fungus Symbiosis: Nutrition, Mutualism and Commensalism. New York: Wiley; 1979. p. 33-52.
29. Harrington TC, Aghayeva DN, Fraedrich SW. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 2010;111:337-61. [DOI]
30. Kasson MT, Wickert KL, Stauder CM, Macias AM, Berger MC, Simmons DR, Short DPG, DeVallance DB, Hulcr J. Mutualism with aggressive wood-degrading Flavodon ambrosius (Polyporales) facilitates niche expansion and communal social structure in Ambrosiophilus ambrosia beetles. Fungal Ecol 2016;23:86-96. [DOI]
31. Lin YT, Shih HH, Hulcr J, Lin CS, Lu SS, Chen CY. Ambrosiella in Taiwan including one new species. Mycoscience 2017;58:242-52. [DOI]
32. Agnello AM, Breth DI, Tee EM, Cox KD, Villani SM, Ayer KM, Wallis AE, Donahue DJ, Combs DB, Davis AE, et al. Xylosandrus germanus (Coleoptera: Curculionidae: Scolytinae) occurrence, fungal associations, and management trials in New York apple orchards. J Econ Entomol 2017;110:2149-64. [DOI]