1 Korean Agricultural Culture Collection, Agricultural Microbiology Division, National Institute of Agricultural Sciences, RDA, Wanju 55365, Korea
2 Hankuk Fermentation Co Ltd, Suji-myeon, Namwon-si, Jeollabuk-do, 55791, Korea
†These authors contributed equally to this work as co-first authors.
*Correspondence to funguy@korea.kr
Korean Journal of Mycology (Kor J Mycol) 2025 March, Volume 53, Issue 1, pages 47-56.
https://doi.org/10.4489/kjm.2025.53.1.6
Received on February 20, 2025, Revised on March 20, 2025, Accepted on March 20, 2025, Published on Mar 31, 2025.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
The Nuruk starter culture has long been used during fermentation to prepare rice wine in Korea. They host several microorganisms that inhabit them and multiply naturally. In the present study, we report the occurrence of the yeast-like fungus Dothiora, in Nuruk samples and on the outer wall of a Nuruk fermentation room in Namwon-si, Jeollabuk-do, South Korea. The strain was designated as KACC 410160, and its growth was observed ranging from temperatures 5°C to 40°C, with optimum growth at 25°C. The colonies were slimy, yeast-like, and spread with characteristic dark (brown to black) velvety textures observed on malt extract agar, potato dextrose agar, oatmeal agar, glucose peptone yeast extract agar, and yeast malt peptone agar. The strain formed brown, septate hyphae on malt extract agar, undergo vegetative reproduction by budding, and produce conidia by conidiogenous cells. A maximum-likelihood phylogenetic tree based on the combined internal transcribed spacer (ITS) and large subunit (LSU) sequences revealed that the strain clustered with D. infuscans FMR 16326T with a bootstrap value of 100%. Based on its morphological, physiological, and molecular characteristics, strain KACC 410160 was identified as D. infuscans. To our knowledge, this is the first report of Dothiora species inhabiting Nuruk in Korea.
Black yeast-like fungi, Dothiora infuscans, Fermentation, Nuruk
Nuruk is a traditional Korean fermentation starter used to manufacture starch-based alcoholic beverages. Conventionally, cereal grains, including wheat, barley, rice, soybean, rye, and millet, have been predominantly used for the preparation of starter culture Nuruk, and the grains are fermented using environmentally derived microorganisms such as mold, yeast, and bacteria [1,2]. The starter material Nuruk hosts several fungi, including Aspergillus, Lichtheimia, Mucor, Rhizomucor, and Rhizopus [1,3,4]. In addition to filamentous fungal species, Nuruk has also been reported to contain numerous yeasts, such as Kluyveromyces, Pichia, Rhodotorula, Saccharomyces, Saccharomycopsis, and Torulopsis, as well as different kinds of bacteria from the genera Bacillus, Cronobacter, Enterococcus, and Pediococcus [4–7]. In the past, black-colored yeast-like fungi have been isolated from extreme niches, such as the Antarctic desert [8] and industrial warehouses with blackened or darkened walls [9]. Characteristically, these yeasts have thick, melanized cell walls and the ability to produce exopolysaccharides, which help them adapt well to harsh environments [8].
A dark-colored fungus Dothiora was introduced in 1849 [10] with D. pyrenophora as the type species. These fungi produce Dothichiza asexual morphs in culture [11]. Species of Dothiora have been isolated from dead branches of trees, woody plants, dead leaves, and fruits, indicating their probable saprophytic nature with a marginal possibility of acting as a weak plant pathogen. This may be due to the fact that the natural ecological niche of a species can play an important role in shaping its potential pathogenicity. The asexual morph of Dothiora is dothichiza-like and characterized by pycnidial conidiomata, phialidic conidiogenous cells, and hyaline and aseptate conidia, forming a hormonema-like synasexual morph [11–14]. In the Dothiora sexual morph, immersion in erumpent ascostromata, which lack pseudoparaphyses, can be observed. The spore count is usually eight or higher with bitunicate asci, hyaline to yellow or light brown in color, and single septate or muriform ascospores [11,14].
In 2022, black yeast-like fungi were observed inside the fermentation starter, Nuruk, in Korea, causing spoilage. Furthermore, the fungus adhered to the grains and could not be removed from Nuruk. The same fungus was also found on the exterior walls of a Nuruk fermentation room. Therefore, we aimed to identify these species to determine an appropriate control method.
Nuruk samples and samples from outside walls of the Nuruk fermentation room were collected from Hankuk Fermentation Co., Ltd., Namwon-si, Jeollabuk-do, in August 2022 (Figs. 1A and B). We isolated the fungus from both the Nuruk and walls using two different isolation methods: direct plating on malt extract agar (MEA) with chloramphenicol (0.05 g/1000 mL) and dilution plating on dichloran 18 % glycerol agar as well as dichloran rose-bengal chloramphenicol agar (DRBC) at 25°C after 3 days [15,16]. Following isolation, the fungal strain was purified and stored at 4°C on MEA slant until further experiments. The strain was deposited at the Korean Agricultural Culture Collection (KACC) and the sequences were submitted to NCBI GenBank.
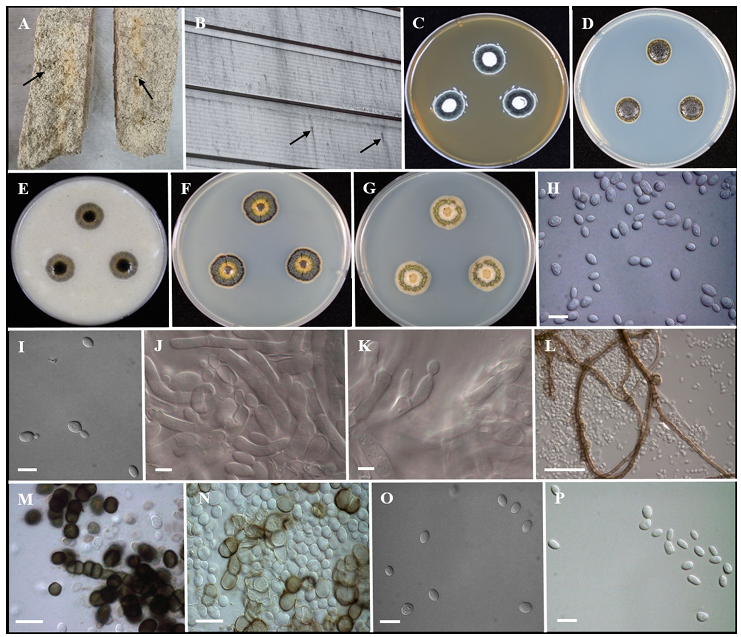
Fig. 1. Morphology of Dothiora infuscans (KACC 410160). A, B: Fungal presence on Nuruk & Wall of the warehouse (black arrows). C–G: Colonies grown on MEA, PDA, OA, GPYA and YM media after 7 days at 25°C from left to right. H, I: Conidia undergoing budding. J, K: Conidiogenous cell. L: Brown hyphae forming on MEA. M, N: Muriform cells. O, P: Conidia. Scale bars: H, I = 10 µm; J, K = 5 µm; L = 50 µm; M, N = 20 µm; O, P = 10 µm. MEA, malt extract agar; PDA, potato dextrose agar; OA, oatmeal agar; GPYA, glucose peptone yeast extract agar; YM, yeast malt peptone agar.
Study of morphological characteristics was carried out on MEA, PDA (potato dextrose agar), OA (oatmeal agar), GPYA (glucose peptone yeast extract agar) and YM (yeast malt peptone agar) after 7 days of incubation at 25°C. The cell morphology of the strain grown on MEA was observed using a Zeiss AXIO Imager A1 microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany), differential interference contrast (DIC) illumination, and a digital camera (AxioCam ICc 3, Carl Zeiss) (Fig. 1). Growth at various temperatures (5, 10, 15, 20, 25, 30, 35, and 40°C) was determined after 7 days of cultivation on MEA and GPYA.
Genomic DNA was extracted from 3-day-old cultures grown on MEA. Extraction was performed using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Internal transcribed spacer (ITS) region and nuclear large subunit (nrLSU) rDNA D1-D2 domain (26S) gene sequences were amplified by PCR using the universal primers ITS1/ITS4 and LR0R/LR7 [17]. DNA sequencing was performed by Macrogen (Seoul, South Korea) using the above primers. Phylogenetic analyses were performed based on ITS and nrLSU sequences. The sequences produced in this study were combined with reference species retrieved from National Center for Biotechnology Information (NCBI) GenBank (Supplementary Table 1), using MAFFT v.7.1. Alignment was followed by concatenation of the gene sequences, and maximum likelihood method-based phylogenetic analyses were conducted in IQ-tree 2.1.2, with 10,000 ultrafast bootstrap approximations [18,19] and visualized in MEGA XI.
Supplementary Table 1. GenBank accession numbers of strains used in phylogenetic analysis
| Species name | Strain number | GenBank accession numbers | |
|---|---|---|---|
| ITS | LSU | ||
| Dothiora agapanthi | CPC 20600T | KU728498 | KU728537 |
| Dothiora aloidendri | CBS 146775T | NR171992 | NG074489 |
| Dothiora buxi | MFLU 15-3404T | KX765294 | KX765295 |
| Dothiora bupleuricola | CBS 112.75T | KU728499 | KU728538 |
| Dothiora cactacearum | CBS 142492T | KY929143 | KY929176 |
| Dothiora cannabinae | CBS 737.71T | AJ244243 | MH872076 |
| Dothiora ceratoniae | CBS 477.69T | KF251151 | KF251655 |
| Dothiora elliptica | CBS 736.71T | KU728502 | KU728541 |
| Dothiora europaea | CBS 739.71T | MH860321 | MH872077 |
| Dothiora infuscans | FMR 16326T | LT993342 | LT993345 |
| Dothiora laureolae | CBS 744.71T | KU728503 | KU728542 |
| Dothiora maculans | CBS 299.76 | KU728504 | KU728543 |
| Dothiora oleae | CBS 615.72T | KU728511 | MH872293 |
| Dothiora phaeosperma | CBS 870.71 | KU728512 | KU728550 |
| Dothiora phillyreae | CBS 473.69T | KU728513 | EU754146 |
| Dothiora prunorum | CBS 933.72T | AJ244248 | KU728551 |
| Dothiora pyrenophora | CPC 30632NT | KY929145 | KY929178 |
| Dothiora rhamni-alpinae | CBS 745.71T | MH860327 | MH872082 |
| Dothiora sorbi | CBS 742.71 | KU728514 | KU728552 |
| Dothiora viburnicola | CBS 274.72T | KU728515 | KU728554 |
| Sydowia polyspora | CBS 128.64T | AJ244262 | MH870017 |
ITS: internal transcribed spacer; LSU: large subunit.
CBS, Culture collection of the Westerdijk Fungal Biodiversity Institute, Netherlands; CPC, Culture collection of Pedro Crous, housed at CBS; FMR, Culture Collection of the Faculty of Medicine at Rovirai Virgili University, Reus, Spain; MFLU, MFLUCC, MaeFah Luang University, Chiang Rai, Thailand; NT, ex-neotype culture; T, extype culture.
Nuruk samples were prepared from wheat and rice at Hankuk Fermentation Co., Ltd. (Namwonsi, Jeollabuk-do, South Korea). A black-colored fungus was visible on Nuruk made from rice flour, but was not found on Nuruk made using wheat grain (Fig. 1A). The fungus entered cracks on the surface of Nuruk and spread extensively inside the Nuruk. The fungus was sticky and adhered strongly to the Nuruk, making it impossible to remove using a spray of air pressure. When the fungus enters liquid fermentation, it floats on the surface, and the alcoholic beverage turns turbid or dirty, making it unusable. Therefore, all Nuruk samples that showed the presence of a sticky black fungus were discarded by the manufacturers. We attempted to identify the spoilage-causing fungus of Nuruk based on its morphology, physiology, and molecular features. The fungus was found to be dimorphic, had characteristics of both yeast and f ilamentous fungi, and was designated as KACC 410160.
Culture characteristics [7 days, 25°C] – Colonies were creamy, slimy, yeast-like, spreading, with blackish green velvety mycelium reaching 16–17 mm on MEA surface, reverse yellowish orange. On PDA and OA, black-colored colonies that were sticky in nature attained 15–16 mm and were reverse black. On GPYA, colonies were dark brown at the center surrounded by a light yellowish-brown layer, followed by a green velvety ring and white mycelium at the margin reaching 14–15 mm. On YM, colonies were brown at the center surrounded by a creamy layer, followed by a light green circle and white mycelia at the margin reaching 14–15 mm (Figs. 1C–G). Mycelium was composed of smooth, thin-walled, septate hyphae, 5–7 μm wide, later becoming thick-walled, increasing the number of septa and the volume of their cells to give them a moniliform appearance, and finally the hyphae turned dark brown & observed the presence of muriform cells. Conidiophores were micronematous, reduced to conidiogenous cells, mostly intercalary, producing conidia laterally and rapidly. Conidia were holoblastic, and solitary, but attached by a mucilaginous substance; mostly aseptate, smooth, ellipsoidal or irregular (few) in shape and size ranges from 5.3–6.8 µm in length and 3.0–3.8 µm in width (Figs. 1H–P). Endoconidial or sexual morphs were not observed. The morphological and culture characteristics of KACC 410160 were consistent with those of D. infuscans and distinct from D. aloidendri (Table 1). Growth on MEA and GPYA media was observed between 5°C and 30°C, with very mild growth at 35°C and no growth at 40°C after 7 days of incubation. This strain showed optimal growth at 25°C (Fig. 2).
Table 1. Comparison of the morphological characteristics of the KACC 410160 with closely related species
| Characteristics | KACC 410160a | D. infuscansTb | D. aloidendriTc | |
|---|---|---|---|---|
| Colony | MEA | Colonies were creamy, slimy, yeast-like, spreading, with blackish green velvety mycelium reaching 16–17 mm after 1 week (25°C). Reverse – yellowish orange | Colonies 27–29 mm at 3 weeks (25°C), center flat and light yellow (4A5) and successively greyish yellow (4B5), pale yellow (4A3) and reddish yellow (4A7) towards the edge. Reverse – light yellow (4A4) | Colonies 40 mm at 2 weeks (25°C), flat and spreading, scant to moderate aerial mycelium with smooth, lobate margins. On MEA surface sepia, Reverse -isabelline |
| PDA | Colonies were black in color and sticky in nature attained 15–16 mm after 1 week (25°C). Reverse – Black | Colonies 28–29 mm at 3 weeks (25°C), center flat and slimy with sulcate edges, centre yellowish brown (5D8), edges brownish black (6H8) and margins light yellow (3A5). Reverse – centre was light orange (5A4), edges brownish grey (5E2), and margins were pale yellow (4A3). | Surface iron-grey Reverse-olivaceous-grey | |
| OA | After 7 days at 25°C, Colonies attained 15–16 mm, the surface was found to be black with sticky appearance. Reverse – Black | Colonies 6–7 mm at 3 weeks (25°C), slightly elevated, compact, irregular margins and colored blackish blue (20F8), yeast-like conidia were abundant; Reverse – centre blackish brown (6G8) and edges brownish orange (5C3), no diffusible pigment | Surface olivaceous-grey. | |
| Conidiophores | Conidiophores m icronematous, reduced to conidiogenous cells, mostly intercalary, producing conidia on lateral and short | Conidiophores micronematous, reduced to conidiogenous cells, mostly intercalary, producing conidia on lateral, short to long conic-truncate denticles, with 1–3 per conidiogenous cell | Conidiophores reduced to conidiogenous cells lining the the inner cavity, hyaline, smooth, ampulliform to doliiform, phialidic, 6–9 × 5–7 μm. | |
| Conidia | Holoblastic, solitary, but attached to one another by a mucilaginous substance; mostly aseptate, smooth and thin to thick-walled, hyaline, becoming dark brown | Holoblastic, solitary, but linked to each other by a mucilaginous substance; mostly aseptate, smooth-and thin to thick-walled, hyaline, becoming dark brown, thick-walled, roughened and mostly 1-septate, occasionally 2–3-septate. | Solitary, straight, s ubcylindrical, aseptate, guttulate, hyaline, smooth, thin-walled, apex obtuse, tapering at base to truncate hilum, 1–1.5 μm diam | |
| Shape | Ellipsoidal or irregular (few) | Globose, ellipsoid or i rregularly-shaped | ||
| Size (μm) | 5.3–6.8 × 3.0–3.8 | 8–9 × 4–5 | (10- -)12–13(–14) × (3–)4 | |
| Host/Substrate | Nuruk/ Blackened wall of an Nuruk fermentation room | Blackened wall of an industrial warehouse | On leaves of Aloidendron dichotomum (A sphodelaceae) | |
| Country | South Korea | Spain | South Africa |
a Fungal strain investigated in this study; b source of description [9]; c source of description [27]; T Type strain.
MEA: malt extract agar; PDA: potato dextrose agar; OA: oatmeal agar.
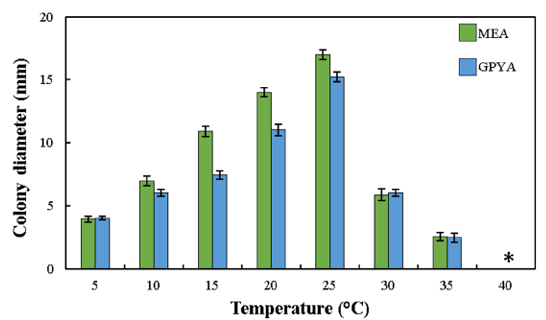
Fig. 2. Effect of temperature on the growth of Dothiora infuscans (KACC 410160) on MEA and GPYA media at 5–40°C after 7 days. *no visible growth. MEA, malt extract agar; GPYA, glucose peptone yeast extract agar.
Partial nucleotide sequences of the ITS region (511 bp) and LSU (837 bp) genes were obtained from the isolated strain to analyze the molecular and phylogenetic relationships. BLASTn analyses of the sequences revealed that the KACC 410160 sequence of 26S rRNA (LSU) and ITS showed 100% similarity to D. infuscans FMR 16326T. A concatenated sequence alignment of two loci (ITS and LSU) from KACC 410160, along with 20 ex-type strains and an outgroup (Sydowia polyspora CBS128.64) in GenBank contained 1,348 characters, including gaps (gene boundaries of ITS:1–511 and LSU: 512–1,348), of which 83 characters were parsimony-informative, 151 were distant sites, and 1,187 were constant. The best-fit model TIM2e+I+G4 was selected for maximum likelihood (ML) phylogenetic analysis. The phylogenetic tree revealed that the strain clustered with D. infuscans FMR 16326Twith a bootstrap value of 100% (Fig. 3). The sequences were deposited in NCBI GenBank under the accession no. for PV156093 (ITS) and PV156094 (LSU), respectively.
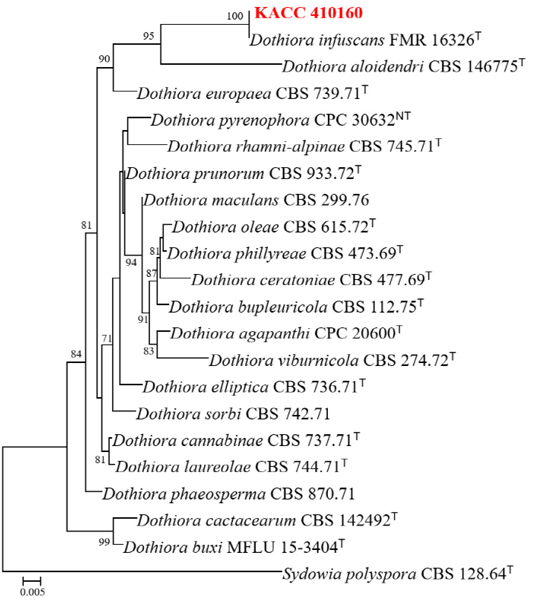
Fig. 3. Phylogenetic tree inferred by concatenated ITS & LSU region of Dothiora species. Tree was constructed using maximum likelihood method, TIM2e+I+G4 model with bootstrap value of 1000. The unrecorded species are in red color. Bootstrap values ≥70 are presented at the nodes. Ex-type strains are denoted by T. The species S. polyspora was used as the outgroup. ITS, internal transcribed spacer; LSU, large subunit.
The black-colored fungus that causes spoilage in Nuruk was identified as D. infuscans based on its molecular and morphological characteristics. We observed 100 % similarity in the rDNA LSU and ITS sequences between the spoilage fungus and the type strain D. infuscans FMR 16326T. Furthermore, the environment in which the Nuruk spoilage fungus occurs is very similar to that in which the ex-type strain was reported to occur on the blackened wall of an industrial warehouse [9]. Several dark-colored or black yeasts from the phylum Ascomycota have been reported to adapt and grow under a wide range of environmental conditions. Black yeasts belonging to the order Dothideales were commonly found in plant materials [8]; however, in our case, we isolated strain KACC 410160 from Nuruk and the exterior wall of the Nuruk fermentation room. Melanized fungi living in extreme environments were mostly yeast-like or meristematic for at least part of their life cycle [20]. Several factors contribute to the ecological sustainability of such black fungi. Among them are the ability to produce pigments such as melanin and carotene, the formation of thick cell walls, yeast-like growth phases, and diverse modes of conidiogenesis. Pigmentation in black fungi helps to exhibit resistance to UV radiation and aids in thermo- and osmotolerance. Other significant traits include hydrophobicity and the ability to produce extracellular polysaccharides, which facilitate strong adhesion to substrates, siderophores, and secondary metabolites with acidic or alkaline properties. Strain KACC 410160 displayed a sticky texture, likely due to the production of exopolysaccharides, and exhibited tolerance to low temperatures.
Dothiora classification was mainly based on sexual morphs. Currently, while only the sexual morph is known for 40 species of Dothiora whereas 15 species are known only through their asexual morphs, and no information was available for eight species. Information on both asexual and sexual morphs was available for only eight species. Despite the currently available molecular data for 34 species, comparative analyses indicated that, in only four species, molecular data correlated with data from sexual/asexual morphs or culture-based studies. A checklist of 72 accepted Dothiora species compiled by Senwanna et al. [21] includes detailed information from the Index Fungorum [22], MycoBank [23], and published literature.
Ecologically, Dothiora species have been reported to be associated with 38 plant host families, and only D. infuscans has been reported from the blackened wall of an industrial warehouse [9]. The endophytic Dothiora sp. produces cytotoxic compounds that act against cancer cell lines [24]. In Korea, D. cannabinae (KACC 37087) has been reported as an unrecorded yeast isolated from a soil sample [25], whereas Dothiora sp. was identified as an endophytic fungus isolated from Taxus cuspidata inhabiting Mount Hallasan, Korea [26].
In this study, we characterized a black fungus originating from Nuruk and the wall of a warehouse. This strain was identified as D. infuscans based on morphological and phylogenetic analyses. The fungus causes spoilage of Nuruk. Additional studies are needed to control its growth in Nuruk.
The authors declare that there is no potential conflict of interest
We thank Dr. Soon Ok Kim and Dr. Chorong Ahn for reviewing the manuscript. We sincerely thank Nan-Hee Lee, Seon-Hee Kim, and Eun-Ha Yuk for their assistance in the laboratory.
This study was supported by the grant (PJ01728601) from the Rural Development Administration.
1. Yang S, Lee J, Kwak J, Kim K, Seo M, Lee YW. Fungi associated with the traditional starter cultures used for rice wine in Korea. J Korean Soc Appl Biol Chem 2011;54:933-43. [DOI]
2. Yang S, Choi SJ, Kwak J, Kim K, Seo M, Moon TW, Lee YW. Aspergillus oryzae strains isolated from traditional Korean Nuruk: fermentation properties and influence on rice wine quality. Food Sci Biotechnol 2013;22:425-32. [DOI]
3. Song SH, Lee C, Lee S, Park JM, Lee HJ, Bai DH, Yoon SS, Choi JB, Park YS. Analysis of microflora profile in Korean traditional nuruk. J Microbiol Biotechnol 2013;23:40-6. [DOI]
4. Kim M, Kim S, Ha BS, Park HY, BaeK SY, Yeo SH, Ro HS. Diversity, saccharification capacity, and toxigenicity analyses of fungal isolates in Nuruk. Kor J Mycol 2014;42:191-200. [DOI]
5. Carroll E, Trinh TN, Son H, Lee YW, Seo JA. Comprehensive analysis of fungal diversity and enzyme activity in nuruk, a Korean fermenting starter, for acquiring useful fungi. J Microbiol 2017;55:357-65. [DOI]
6. Lee JE, Lee AR, Kim H, Lee E, Kim TW, Shin WC, Kim JH. Restoration of traditional Korean Nuruk and analysis of the brewing characteristics. J Microbiol Biotechnol 2017;27:896-908. [DOI]
7. Bal J, Yun SH, Yeo SH, Kim JM, Kim DH. Metagenomic analysis of fungal diversity in Korean traditional wheat-based fermentation starter nuruk. Food Microbiol 2016;60:73-83. [DOI]
8. Selbmann L, Hoog S, Mazzaglia A, Friedmann EI, Onofri S. Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Stud Mycol 2005;51:1-32.
9. Crous PW, Wingfield MJ, Burgess TI, Hardy GESJ, Gené J, Guarro J, Baseia IG, García D, Gusmão LFP, Souza-Motta CM, et al. Fungal Planet description sheets: 716-784. Persoonia 2018;40:240-393. [DOI]
10. Fries E. Eliae Fries Summa vegetabilium Scandinaviae, seu Enumeratio systematica et critica plantarum quum cotyledonearum, tum nemearum inter Mare Occidentale et Album, inter Eidoram et Nordkap, hactenus lectarum, indicata simul distributione geographica … Accedunt expositio systematis plantarrum morphologici, comparatio vegetationis adjacentium regionum, definitiones specierum in Kochii Synopsi florae germanicae et nemearum monographiis haud obviarum L. aliter expositarum. Holmiae: A. Bonnier; 1849.
11. Crous PW, Groenewald JZ. The genera of fungi – G 4: Camarosporium and Dothiora. IMA Fungus 2017;8:131-52. [DOI]
12. Sivanesan A. The bitunicate ascomycetes and their anamorphs. Vaduz: J. Cramer; 1984.
13. Crous PW, Groenewald JZ. They seldom occur alone. Fungal Biol 2016;120:1392-415. [DOI]
14. Thambugala KM, Ariyawansa HA, Li YM, Boonmee S, Hongsanan S, Tian Q, Singtripop C, Bhat DJ, Camporesi E, Jayawardena R, et al. Dothideales. Fungal Divers 2014;68:105-58. [DOI]
15. Hong SB, Kim DH, Lee M, Baek SY, Kwon SW, Samson RA. Taxonomy of Eurotium species isolated from meju. J Microbiol 2011;49:669-74. [DOI]
16. Hong SB, Kim DH, Kim SH, Bang N, Kwon SW. Identification of black Aspergillus strains isolated from meju. Kor J Mycol 2013;41:132-5. [DOI]
17. White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, editors. PCR protocols: A guide to methods and applications. New York: Academic Press; 1990. p. 315-22. [DOI]
18. Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 2018;35:518-22. [DOI]
19. Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 2015;32:268-74. [DOI]
20. de Hoog GS, Zalar P, Urzì C, de Leo F, Yurlova NA, Sterflinger K. Relationships of dothideaceous black yeasts and meristematic fungi based on 5.8S and ITS2 rDNA sequence comparison. Stud Mycol 1999;43:31-7.
21. Senwanna C, Hongsanan S, Khuna S, Kumla J, Yarasheva M, Gafforov Y, Abdurazakov A, Suwannarach N. Insights into the molecular phylogeny and morphology of three novel Dothiora species, along with a worldwide checklist of Dothiora. Front Cell Infect Microbiol 2024;14:1367673. [DOI]
22. Index Fungorum Partnership. Index Fungorum [Internet]. Place unknown: Index Fungorum Partnership; 2025 [cited 2025 Feb 2]. Available from http://www.indexfungorum.org.
23. International Mycological Association, Westerdijk Fungal Biodiversity Institute. MycoBank [Internet]. Utrecht: International Mycological Association, Westerdijk Fungal Biodiversity Institute; 2025 [cited 2025 Feb 2]. Available from https://www.mycobank.org.
24. Pérez-Bonilla M, González-Menéndez V, Pérez-Victoria I, de Pedro N, Martín J, MoleroMesa J, Casares-Porcel M, González-Tejero MR, Vicente F, Genilloud O, et al. Hormonemate derivatives from Dothiora sp., an endophytic fungus. J Nat Prod 2017;80:845-53. [DOI]
25. Han JH, Oh HJ, Lee SE, Kim MK. Isolation of ten unrecorded yeasts from soil in Korea. J Species Res 2021;10:336-43.
26. Cha JE, Park H, Eom AH. Species diversity of endophytic fungi isolated from Taxus cuspidata inhabiting Mt. Hallasan, Korea. Kor J Mycol 2023;51:419-28.
27. Crous PW, Cowan DA, Maggs-Kölling G, Yilmaz N, Larsson E, Angelini C, Brandrud TE, Dearnaley JDW, Dima B, Dovana F, et al. Fungal Planet description sheets: 1112-1181. Persoonia 2020;45:251-409. [DOI]