Korean Journal of Mycology (Kor J Mycol) 2023 December, Volume 51, Issue 4, pages 428. https://doi.org/10.4489/KJM.20230043
Received on October 31, 2023, Revised on December 15, 2023, Accepted on December 20, 2023.
Copyright © The Korean Society of Mycology.
This is an Open Access article which is freely available under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC) (https://creativecommons.org/licenses/by-nc/4.0/).
INTRODUCTION
Endophytic fungi are symbiotic with plants [1]. They live in plant tissues without causing diseases [2]. These fungi chemically defend their host plants against herbivores, insects, and external pathogens [3,4]. Additionally, they enhance the host plants’ resistance to environmental stressors [5]. Endophytic fungi form symbiotic relationships with a range of plants across different climatic zones [6,7]. Their presence is noted in almost all plants, including bryophytes, ferns, conifers, evergreen broad-leaved trees, and deciduous broad-leaved trees [8-12]. These fungi colonize a variety of plant tissues, from vegetative organs, such as roots, stems, and leaves, to reproductive organs, such as flowers and fruits [7]. The relative abundance, species diversity, and community structure of endophytic fungi can vary based on the specific plant tissue [13,14].
Taxus cuspidata Siebold et Zucc. is an evergreen coniferous tree that is geographically distributed in the Far East of Russia, northeastern China, Japan, the Korean Peninsula, and Jeju Island. T. cuspidata grows at altitudes ranging from 700 to 2,500 meters above sea level from Mt. Sungjeoksan in North Korea to Mt. Hallasan on Jeju Island, South Korea [15]. Various tissues of T. cuspidata such as the stem bark, root bark, fibrous roots, twigs, and leaves, produce the anticancer substance taxol (paclitaxel) [16]. Furthermore, endophytic fungi, such as Pestalotiopsis spp. isolated from T. cuspidata, have been shown to produce taxol [17,18].
In this study, we isolated and identified the endophytic fungal strains from T. cuspidata inhabiting Yeongsil area in Mt. Hallasan, Jeju Island. We attempted to confirm the species diversity and community structure according to the plant tissue parts from where the endophytic fungi were isolated.
MATERIAL AND METHODS
RESULTS AND DISCUSSION
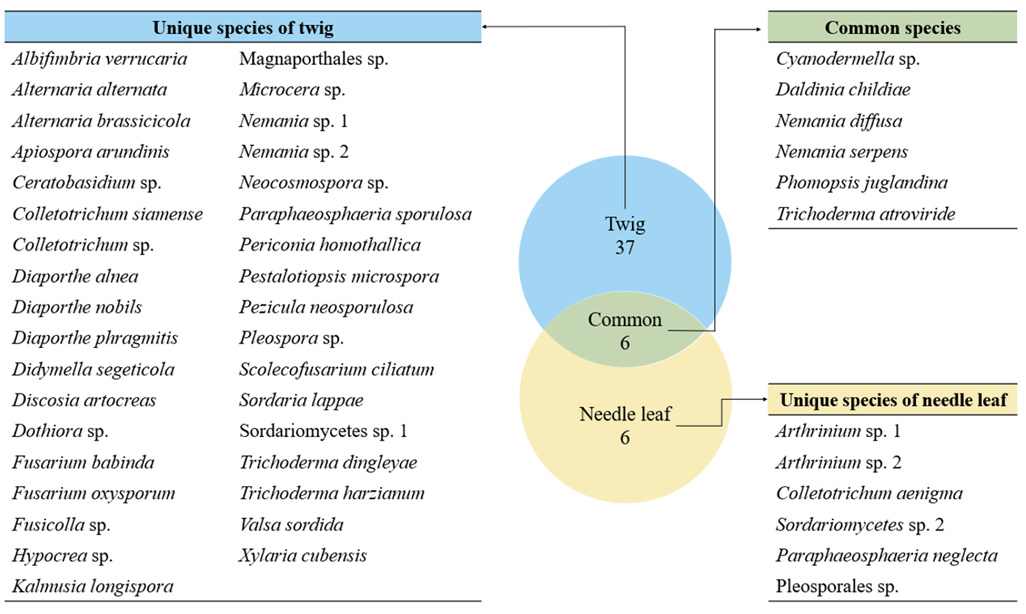
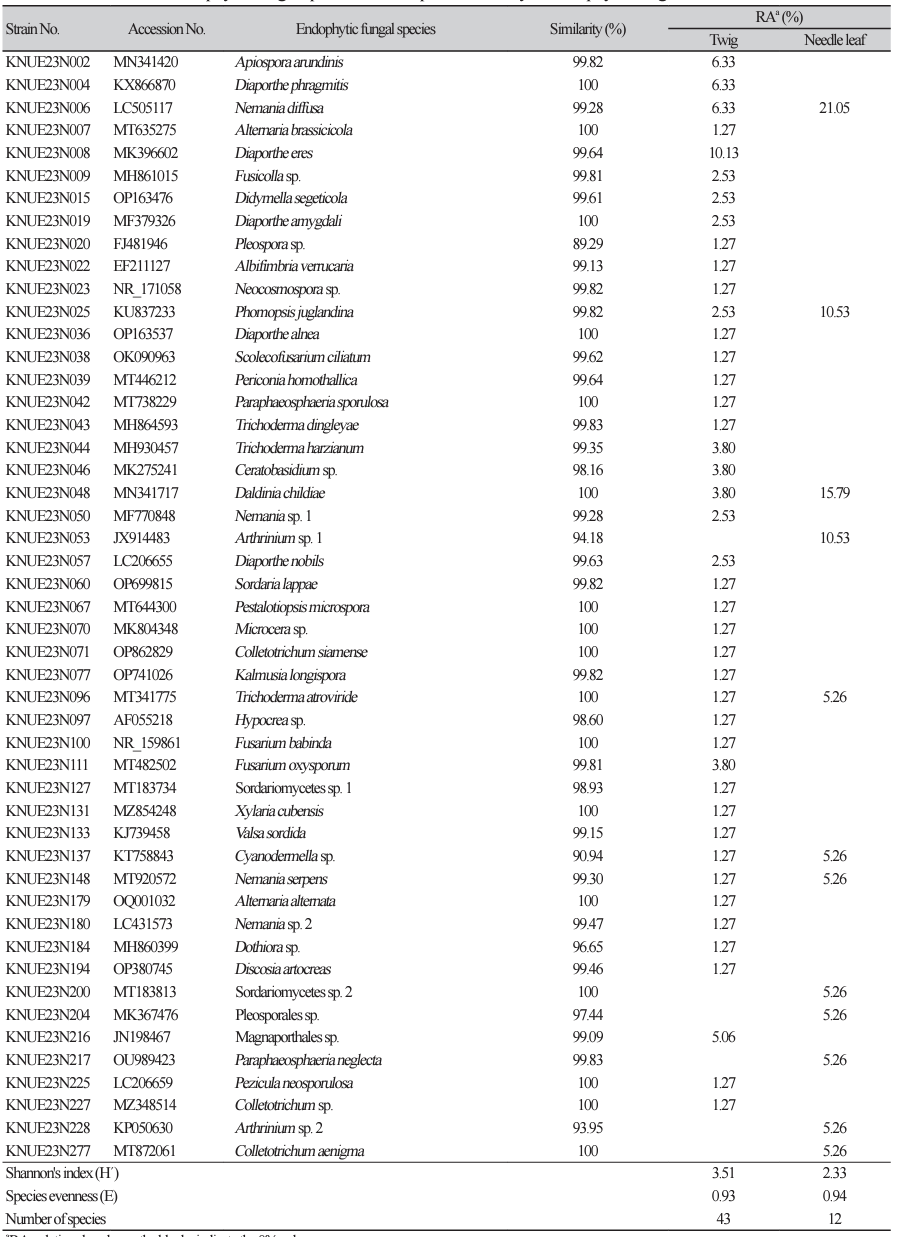
We isolated a total of 98 endophytic fungal strains from the needle leaves and twigs of T. cuspidata. Molecular identification revealed 49 fungal species across 33 genera (Fig. 1). From needle leaves, 12 species representing 10 genera were isolated, while from the twigs, 43 species from 29 genera were isolated (Table 1).
Among the isolated endophytes, only Ceratobasidium sp. belonged to the phylum Basidiomycota, and all other species belonged to the phylum Ascomycota. Six species (Cyanodermella asteris, Daldinia childiae, Nemania diffusa, N. serpens, Phomopsis juglandina, and Trichoderma atroviride) were isolated from both needle leaves and twigs. The remaining species were isolated only from either needle leaves or twigs (Fig. 1). Among the species isolated from needle leaves, N. diffusa showed the highest relative abundance at 21.05%. In twigs, Diaporthe eres showed the highest relative abundance of 10.13%. This suggests that the endophytic fungal communities in needle leaves and twig have distinct species composition, indicating that the plant tissue sites can influence the structure of endophytic fungal community [23].
Of the fungal species isolated, two had not been previously unrecorded in Korea. We describe the morphological characteristics and phylogenetic analysis results for these two fungal strains.
Table 1 The identified endophytic fungal species and the species diversity of endophytic fungal communities.
REFERENCE
Carroll G. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology 1988;69:2-9.
https://doi.org/10.2307/1943154
Gao FK, Dai CC, Liu XZ. Mechanisms of fungal endophytes in plant protection against pathogens. Afr J Microbiol Res 2010;4:1346-51.
Bouton J, Gates R, Belesky D, Owsley M. Yield and persistence of tall fescue in the southeastern coastal plain after removal of its endophyte. Agron J 1993;85:52-5.
https://doi.org/10.2134/agronj1993.00021962008500010011x
Rowan DD, Latch GC, Bacon C, White J. Utilization of endophyte-infected perennial ryegrasses for increased insect resistance. Biotechnology of Endophytic Fungi of Grasses. Boca Raton: CRC Press; 1994. p. 169-83.
https://doi.org/10.1201/9781351070324-12
Arachevaleta M, Bacon CW, Hoveland CS, Radcliffe DE. Effect of the tall fescue endophyte on plant response to environmental stress. Agron J 1989;81:83-90.
https://doi.org/10.2134/agronj1989.00021962008100010015x
Zhang HW, Song YC, Tan RX. Biology and chemistry of endophytes. Nat Prod Rep 2006;23:753-71.
https://doi.org/10.1039/b609472b
Eo JK, Choi JW, Eom AH. Diversity, distribution, and host plant of endophytic fungi: a focus on Korea. Mycobiology 2022;50:399-407.
https://doi.org/10.1080/12298093.2022.2154044
Choi HS, Park H, Eo JK, Eom AH. Unrecorded endophytic fungi isolated from Mnium heterophyllum and Hypnum plumaeforme in Korea: Biscogniauxia petrensis and Cercophora thailandica. Kor J Mycol 2020;48:55-61.
Oh SY, Park KH, Baldrian P, Fong JJ, Kwon HJ, Kim SY, Lim YW. Fungal diversity living in the root and sporophore of the endemic Korean fern Mankyua chejuense. Fungal Ecol 2021;50:101038.
https://doi.org/10.1016/j.funeco.2020.101038
Eo JK, Eom AH. Diversity of foliar endophytic fungi inhabiting coniferous trees in Korea. Kor J Mycol 2018;46:205-11.
Gwon JH, Park H, Lee JC, Choi JW, Eom AH. Unreported endophytic fungi isolated from the leaves of evergreen woody plants in Korea: Muyocopron lithocarpi and Didymocyrtis cladoniicola. Kor J Mycol 2021;49:373-8.
Kim CK, Eo JK, Eom AH. Diversity of foliar endophytic fungi isolated from Lindera obtusiloba in Korea. Kor J Mycol 2012;40:136-40.
https://doi.org/10.4489/KJM.2012.40.3.136
Kumar DSS, Hyde KD. Biodiversity and tissue-recurrence of endophytic fungi in Tripterygium wilfordii. Fungal Divers 2004;17:69-90.
Park H, Jung CR, Eom AH. Species diversity and antifungal activity of endophytic fungi isolated from Angelica gigas Nakai. Kor J Mycol 2021;49:497-505.
Cho MG, Chung JM, Jung HR, Kang MY, Moon HS. Vegetation structure of Taxus cuspidata communities in subalpine zone. J Agric Life Sci 2012;46:1-10.
Fang W, Wu Y, Zhou J, Chen W, Fang Q. Qualitative and quantitative determination of taxol and related compounds in Taxus cuspidata Sieb et Zucc. Phytochem Anal 1993;4:115-9.
https://doi.org/10.1002/pca.2800040307
Kim SU, Strobel G, Ford E. Screening of taxol-producing endophytic fungi from Ginkgo biloba and Taxus cuspidata in Korea. J Appl Biol Chem 1999;42:97-9.
https://doi.org/10.1016/j.jbiosc.2010.06.007
Kumaran RS, Kim HJ, Hur BK. Taxol promising fungal endophyte, Pestalotiopsis species isolated from Taxus cuspidata. J Biosci Bioeng 2010;110:541-6.
Choi JW, Lee JM, Park SY, Eom AH. Acrodontium burrowsianum and Pestalotiopsis humicola: two previously unrecorded fungal species isolated from conifer leaves in Korea. Kor J Mycol 2022;50:311-8.
Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 1993;2:113-8.
https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Liu YJ, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 1999;16:1799-808.
https://doi.org/10.1093/oxfordjournals.molbev.a026092
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870-4.s
https://doi.org/10.1093/molbev/msw054
Singh DK, Sharma VK, Kumar J, Mishra A, Verma SK, Sieber TN, Kharwar RN. Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn. f.: Spatiotemporal and tissue type effects. Sci Rep 2017;7:3745.
https://doi.org/10.1038/s41598-017-03933-0
Samuels GJ, Dodd SL, Lu BS, Petrini O, Schroers HJ, Druzhinina IS. The Trichoderma koningii aggregate species. Stud Mycol 2006;56:67-133.
https://doi.org/10.3114/sim.2006.56.03
Rogers JD. Xylaria cubensis and its anamorph Xylocoremium flabelliforme, Xylaria allantoidea, and Xylaria poitei in continental United States. Mycologia 1984;76:912-23.
https://doi.org/10.1080/00275514.1984.12023929
Hashemi SA, Khodaparast SA, Zare R, Elahinia SA. Contribution to the identification of Xylaria species in Iran. Rostaniha 2014;15:153-66.
Fan NW, Chang HS, Cheng MJ, Hsieh SY, Liu TW, Yuan GF, Chen IS. Secondary metabolites from the endophytic fungus Xylaria cubensis. Helv Chim Acta 2014;97:1689-99.
https://doi.org/10.1002/hlca.201400091
Klaiklay S, Rukachaisirikul V, Sukpondma Y, Phongpaichit S, Buatong J, Bussaban B. Metabolites from the mangrove-derived fungus Xylaria cubensis PSU-MA34. Arch Pharm Res 2012;35:1127-31.
https://doi.org/10.1007/s12272-012-0701-y
Macías-Rubalcava ML, Sánchez-Fernández RE. Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World J Microbiol Biotechnol 2017;33:1-22.
https://doi.org/10.1007/s11274-016-2174-5
Fu Y, Li X, Yuan X, Zhang Z, Wei W, Xu C, Song J, Gu C. Alternaria alternata F3, a novel taxol-producing endophytic fungus isolated from the fruits of Taxus cuspidata: isolation, characterization, taxol yield improvement, and antitumor activity. Appl Biochem Biotech 2023;2023:1-24.
https://doi.org/10.1007/s12010-023-04661-0
Gill H, Vasundhara M. Isolation of taxol producing endophytic fungus Alternaria brassicicola from non-Taxus medicinal plant Terminalia arjuna. World J Microbiol Biotechnol 2019;35:1-8.
https://doi.org/10.1007/s11274-019-2651-8
Mosquera-Espinosa AT, Bayman P, Prado GA, Gómez-Carabalí A, Otero JT. The double life of Ceratobasidium: orchid mycorrhizal fungi and their potential for biocontrol of Rhizoctonia solani sheath blight of rice. Mycologia 2013;105:141-50.
https://doi.org/10.3852/12-079
Fisher PJ, Petrini OSBC, Sutton BC. A comparative study of fungal endophytes in leaves, xylem and bark of Eucalyptus in Australia and England. Sydowia 1993;45:338-45.
Win PM, Matsumura E, Fukuda K. Diversity of tea endophytic fungi: cultivar-and tissue preferences. Appl Ecol Environ Res 2018;16:677-95.
https://doi.org/10.15666/aeer/1601_677695
Eo JK, Lee BH, Eom AH. Diversity of endophytes isolated from Thuja koraiensis Nakai in the Korean Peninsula. Kor J Mycol 2016;44:113-7.
https://doi.org/10.4489/KJM.2016.44.2.113
Shahzad R, Khan AL, Bilal S, Asaf S, Lee IJ. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front Plant Sci 2018;9:1-10.
https://doi.org/10.3389/fpls.2018.00024
Saikkonen K, Wäli P, Helander M, Faeth SH. Evolution of endophyte–plant symbioses. Trends Plant Sci 2004;9:275-80.
https://doi.org/10.3389/fpls.2018.00024