Section
Arrow bamboo (Pseudosasa japonica [Siebold & Zuccarini] Makino) is a species of bamboo native to East Asia and is mainly distributed in Japan and the southern part of the Korean Peninsula [1]. Bamboo is widely used in food, medicine, and construction. It grows well in different climates and helps maintain the ecosystem as a carbon sink [2,3]. Bamboo is a host plant for a variety of fungi, and research on bambusicolous fungi began in 1845. To date, more than 1,300 species have been described or recorded. [4]. However, there are limited studies on bamboo fungi, for example, soil-borne fungi [5], fungal endophyte [6], etc. in Korea.
Genus Apiospora Sacc. was proposed by Saccardo in 1875 and 142 species have been reported worldwide [7,8]. Morphological characteristics of the genus Apiospora is basauxic conidiogenesis and variously shaped (globose, angular, polygonal, curved, fusiform, navicular) conidia [9]. In 2013, the genus Apiospora was considered a teleomorph of the genus Arthrinium, so synonymized with the genus Arthrinium under the one fungus-one name policy [10]. However, these two genera were separated again in 2021 based on genetic, morphological, and ecological characteristics [9]. Apiospora spp. and Arthrinium spp. were mainly found in plants of the Poaceae, which includes bamboo [10]. Until now a total of 17 species of Apiospora were reported in bamboo in Korea [6]. The purpose of this study was to report on Apiospora pseudosinensis, previously unrecorded fungal species isolated from arrow bamboo in Korea.
Leaves and stems of arrow bamboo used in the study were collected in Gongju-si, Chungcheongnam-do in April 2023. The collected samples were placed in a sealed zipper bag and stored at a low temperature until fungi were separated within 24 h. All samples were cut into 1.5 cm pieces, and surface sterilized by treating them in a 70% ethyl alcohol solution for 1 minute and in a 3% sodium hypochlorite solution for 2 minutes. After surface sterilization the samples were washed with sterilized water for 3 times [11]. The samples were placed on potato dextrose agar (PDA; Difco lab., Detroit, USA) and then cultured at 25℃ in the dark to isolate endophytic fungi. The isolated mycelium was cultured using PDA, and maltose extract agar (MEA; MBcell, Seoul, Korea) media were used for the pure culture of endophytic fungi to observe the colony’s morphological characteristics [12]. Microstructures in the colonies, including spores, were observed under a light microscope (Axio imager A2, Carl Zeiss, Oberkochen, Germany). The unrecorded endophytic fungus used for observation was deposited in the Korean Agricultural Culture Collection (KACC).
Genomic DNA was extracted from the fungus using a HiGene Genomic DNA Prep Kit (BioFACT, Daejeon, Korea) following the manufacturer’s instructions. For molecular identification, DNA from the internal transcripts spacer (ITS) including 5.8S ribosomal DNA, large subunit ribosomal DNA (LSU) including D1 and D2 region, and β-tubulin (TUB2) regions were amplified using primers ITS1F/ITS4 [13,14], LR0R/LR16 [15], Bt2a/Bt2b [16]. Thermal cycling program was an initial denaturation at 95℃ for 2 min, followed by 20 s at 95℃, 40 s at 44℃ (LSU), 50℃ (ITS) or 55℃ (TUB2), and 50 s at 72℃ for 35 cycles and a terminal extension of 72℃ for 5 min. The PCR product was confirmed by electrophoresis using 1.5% agarose gel. DNA sequencing was supplied to SolGent Co., Ltd. (Daejeon, Korea), and the analyzed DNA sequence was then identified based on similarity with the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/) using the basic local alignment search tool (BLAST). Furthermore, a neighbor-joining (NJ) tree was generated by MEGA 7 based on the Kimura-2 parameter distance model with 1,000 bootstrap replicates [17,18].
Apiospora pseudosinensis (Crous) Pintos & P. Alvarado, Fungal Syst. Evol. 7: 207 (2021) [MB#837709]
The colony diameters after 7 d were 30.4×32.2 mm on MEA and 45.3×43.3 mm on PDA. The surface colors were light brownish gray (Munsell color notation: 2.5Y 9/2) to light gambogeish gray (Munsell color notation: 5Y 8/2) in MEA and light brownish gray (Munsell color notation: 2.5Y 9/2) in PDA, Colonies had a texture similar to cotton with irregular margin and no exudate. Hyphae were clearly observed in the filamentous form. The reverse was light gamboge (Munsell color notation: 2.5Y 8/8) to amberish gray (Munsell color notation: 10Y 8/2) in MEA and light gambogeish gray (Munsell color notation: 7.5Y 8/2) in PDA. Conidia measured about 7.4-9.7×5.9-8.7 μm (n=20) were circular or elliptical with dark grayish brown (Munsell color notation: 2.5Y 3/2) and showed no septa inside. Conidiogenous cells are ampulliform or elliptical with hyaline and internal granules exit, gradually turning brownish gray (Munsell color notation: 5Y 6/2) over time (Fig. 1; Table1).
Specimen examined: Gongju-si, Chungcheongnam-do, Korea, 36°20'8.79''N, 127°11'9.72''E, 2023.4.8., isolated from the stem of Pseudosasa japonica, strain KACC 410370, GenBank no. OR687572 (ITS), OR687573 (LSU) and OR715105 (TUB2)
Note: The genus Apiospora lives in various environments, such as ocean, soil, and plants. [8]. In Korea, some unrecorded and new species of Apiospora and Arthrinium have been isolated and reported from the ocean and soil [19-21]. Four species of Apiospora as plant endophytes were reported recently [6], but continuous research is needed when compared to the global records of the Apiospora spp. A. pseudosinensis was previously collected from the Netherlands in 2012 and China in 2022, and all of strains were isolated from bamboo leaves [10,22]. However, we need to gather more information about the host plants and geographical distribution of A. pseudosinensis. In this study, the nucleotide sequences of ITS including 5.8S ribosomal DNA, LSU, and TUB2, were analyzed and used for the molecular identification of A. pseudosinensis. Each sequence was shown all 100% homology with ITS region of A. pseudosinensis (NR 121559), 28S ribosomal DNA of A. pseudosinensis (NG 042784) and TUB2 region of A. pseudosinensis (OP573272) (Fig. 2). A number of secondary metabolite studies have been conducted on Apiospora spp. For example, A. pseudosinensis has been found to produce substances that increase cell proliferation through phosphorylation of estrogen receptor-α. Therefore, this kind of research on the major fungal resource of secondary metabolites leads to industrial and pharmaceutical applications [23,24]. As mentioned above, the the availability of secondary metabolites and the problem of taxonomy are continually discussed, therefore, further taxonomic studies and elucidation of secondary metabolites of Apiospora spp. should be undertaken.
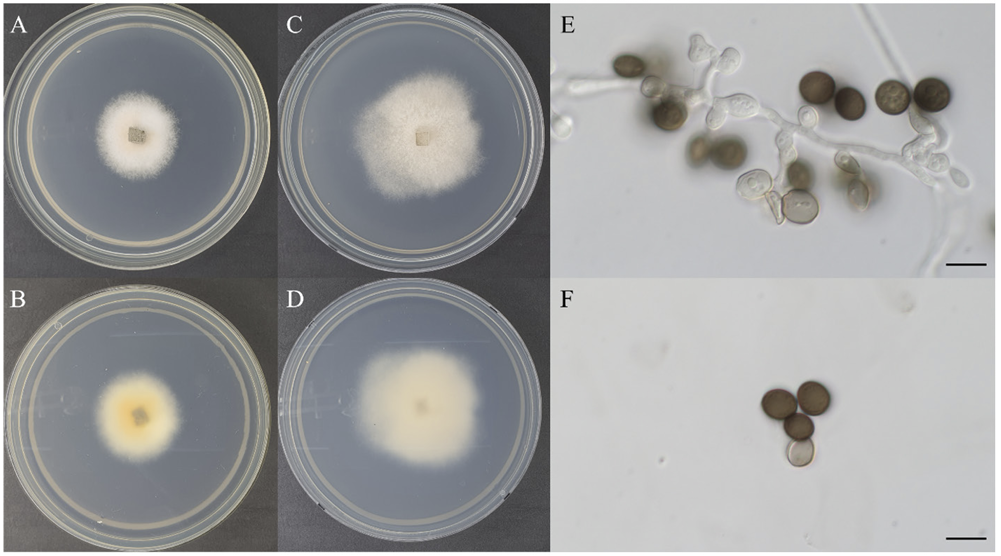
Fig. 1 Cultural characteristics of Apiospora pseudosinensis KACC 410370. A, B: Front and reversed sides of the colony on maltose extract agar (MEA) at 25℃ for 7 days. C, D: Front and reversed sides of the colony on potato dextrose agar (PDA) at 25℃ for 7 days. E: Conidiogenous cells and conidia. F: Conidia. Scale bars: E, F=10 μm.
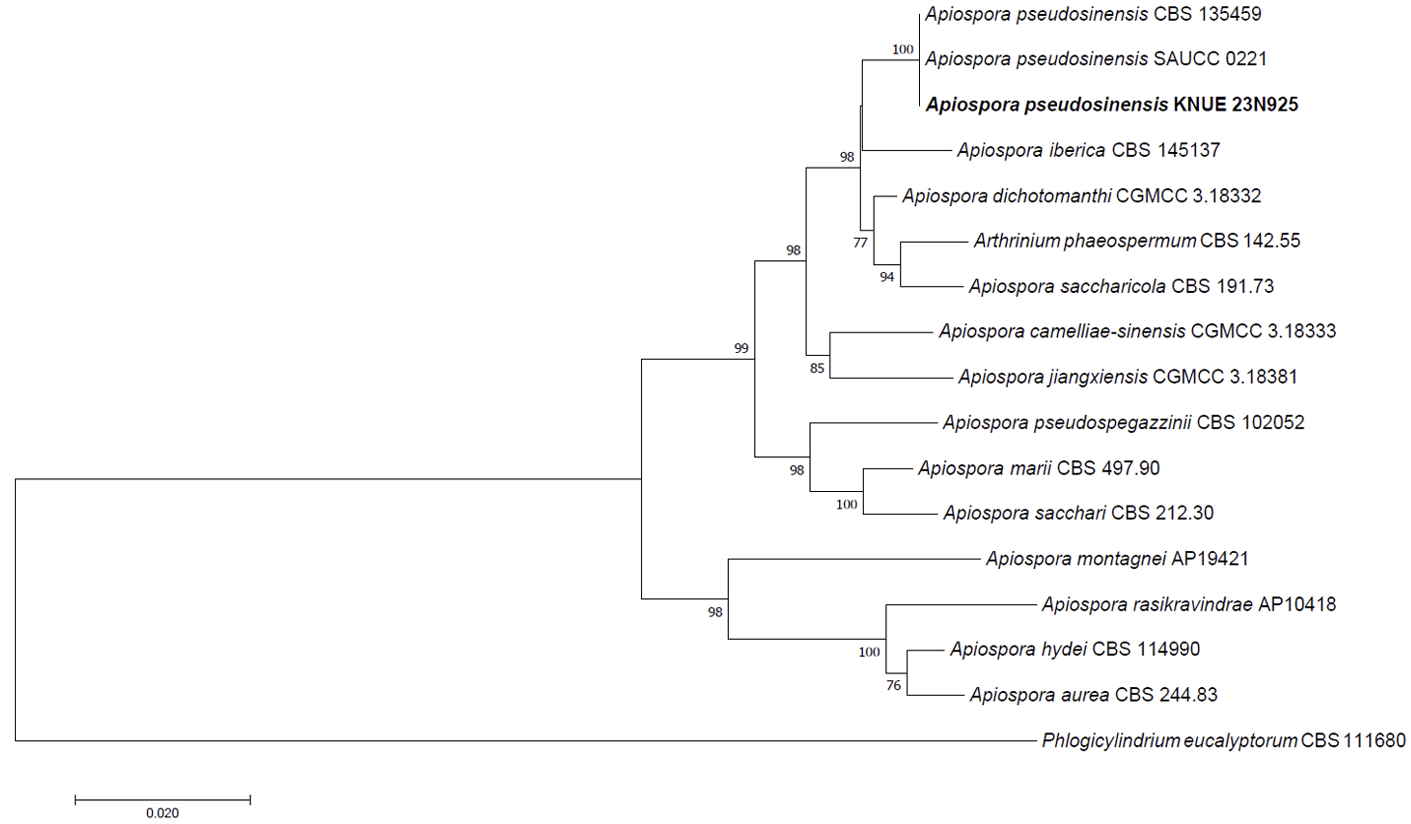
Fig. 2 Neighbor-joining phylogenetic tree of Apiospora pseudosinensis KACC 410370 based on a concatenated alignment of internal transcribed spacer (ITS) including 5.8S ribosomal DNA, large subunit ribosomal DNA (LSU) including D1 and D2 region and β-tubulin (TUB2) DNA sequences. Phlogicylindrium eucalyptorum was used as an out-group. The numbers on the nodes represent bootstrap values upper than 50% (1,000 replicates).
Table 1 Morphological characteristics of Apiospora pseudosinensis KACC 410370.
