Introduction
Arbuscular mycorrhizal fungi (AMF) are symbionts that live in the roots of plants and help host plant improve their water and nutrient uptake, and resistance to pathogens and diseases [1-3]. AMF also protect plants from nutrient deficiencies and abiotic stresses by maintaining the physical properties of the soil [4]. Glomalin-related soil protein is produced by AMF mycelia, which is beneficial for both the soil and AMF, as it contributes to soil aggregation, carbon accumulation, and reduction of erosion [5,6]. Therefore, studies have consistently reported that AMF increase crop yields [7]. However, the use of chemical fertilizers and pesticides is recognized as a serious threat to the use of AMF in agriculture [8,9]. Therefore, AMF can be expected to play an important role in sustainable agriculture [10], and recent efforts have attempted to achieve sustainable farming by utilizing the advantages of AMF. In the United States, India, and Europe, AMF have been used for sustainable agriculture, and fertilizers containing AMF have been commercialized and distributed [11].
In Korea, red pepper (Capsicum annuum L.) is a vegetable that accounts for about 20% of the vegetable cultivation area and is an important cash crop for farmers (Korean Statistical Information Service, http://kisis.kr). Conventional farming has disrupted the ecosystem because of the use of large amounts of chemical fertilizers and pesticides to increase productivity [12]. Agriculture in the future will have to maintain high productivity while maintaining ecological sustainability. Therefore, organic farming, which can repair the disrupted agricultural ecosystem, should be recommended rather than conventional farming, and the introduction of AMF to farming could be considered as one method to improve productivity [13]. The effects of organic farming of red pepper on AMF communities and the glomalin content in the soil has been previously studied [14], and the species diversity of AMF is higher in red pepper grown via organic farming than in conventional farming [15]. In addition, the inoculation effect and method of AMF, according to the amount of phosphate in soil, have been investigated to increase the growth efficiency of red pepper [16]. However, only a few studies have explored the agricultural application of AMF as a biological fertilizer in Korea [14,15,17]. Several problems must be considered when applying AMF in agriculture. Since the AMF strains vary depending on the growth environment, the selection of an AMF strain applicable to farming is necessary. In addition, considering whether the inoculated AMF strain can survive while interacting with native strains in cultivated fields is vital. Therefore, the purpose of this study was to screen AMF strains suitable for the organic farming of red pepper, and to this end, red pepper was inoculated with five AMF species to determine whether AMF inoculum influences red pepper on actual farmland.
Materials and Methods
Production of AMF spores
Field soil samples collected from various regions in Korea were mixed with sterilized sand and trap-cultured for five months using sorghum (Sorghum biocolor (L.) Moench) as a host species. Spores were extracted from cultures using density gradient centrifugation [18]. DNA was extracted from a single spore, and the 18S rDNA was amplified using FLR3-FLR4 primer pairs [19]. The polymerase chain reaction (PCR) products were sequenced and subjected to basic local alignment search tool (BLAST). Based on the morphological and sequence analysis, five AMF species were selected for culture in a single species: Acaulospora longula Spain & N.C. Schenck (AL), Claroideoglomus clarum Thaxt (Gerd. & Trappe) Walker & Koske (CC), Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüßler (CE), Funneliformis mosseae (T.H. Nicolson & Gerd.) C.Walker &A.Schüßler (FM), and Gigaspora gigantea (T.H. Nicol. & Gerd.) Gerd. & Trappe (GG). For single species culture, single spores of each species were inoculated into the roots of sorghum seedlings. The seedlings were planted in pots containing sterilized sand, and then cultured for 4-6 months in a culture room to obtain an adequate amount of spores for each species.
AMF inoculum preparation
The roots of carrots (Daucus carota L.) were cleaned, cut to a width of approximately 5 mm, and sprayed with 200 µL of Rhizobium rhizogenes (KCTC2744) culture suspension [20]. The inoculated roots were then incubated in 1.5% water-agar growth medium in the dark for 4 weeks. The transformed root organs around the cambium were subcultured in a modified White’s (MW) medium containing carbenicillin (500 mg/L). After several generations of subculture, the transformed roots were cut to approximately 3-5 cm before use. Spores of the five species of AMF were extracted from the single species culture soils, surface-sterilized with 2% chloramine T and streptomycin solution, and germinated in water-agar growth medium at 25℃. The germinated spores were selected and transferred to a modified Strullu-Romand growth medium. The germinated spores were placed next to the transformed carrot root organs, which were cut to approximately 1.5 cm×1.5 cm in the MW growth medium. Samples were continuously subcultured every 2-3 months, following which they were used in this study. The roots of the five AMF species and uninoculated control were harvested from the medium and used as the inoculum. The roots were stained with trypan blue to confirm AMF colonization [21]. In addition, equal amounts of roots for each species were mixed and used as a mixture inoculum. The roots were entrapped in alginate beads and air-dried before use [22].
Inoculation and growth of the seedlings in greenhouse
The surface-sterilized red pepper seeds were germinated and inoculated with 2 g of inoculum in a pot filled with sterilized vermiculites. The seedlings were maintained in a greenhouse, watered twice a day, and supplied with Hoagland’s solution once a week. After 10 weeks of growth, the plants were harvested, and the dry weight of roots and shoots were measured.
Transplanting the seedlings to the fields
After 10 weeks of growth in a greenhouse, the seedlings were transplanted to three plots, with 20 replications for each treatment, in cultivated fields where conventional farming was performed, and then cultivated for 10 weeks. The distances between the rows and between plants were 80 cm and 60 cm, respectively. No chemical fertilizers or pesticides were used during the experiments, and the weeds around the pepper seedlings were removed once a week. After 10 weeks, all plants were harvested separately from each other. Fruits, stems, and roots were immediately dried at 50℃ for 72 h, and their dry weights were measured and used for analysis. The soil was dried at 50℃ for 7 days and then analyzed for glomalin content.
Glomalin assay
After 10 weeks of growth in the field, six soil samples from each treatment were randomly collected from the rhizosphere of plants and dried at 50℃ for 7 days, and 1 g of the dried soil sample was used for glomalin determination [23]. Total glomalin was extracted with 50 mM sodium citrate, and the protein concentration was determined using the Bradford assay with bovine serum albumin as the standard, and an enzyme-linked immunosorbent assay (ELISA) with the monoclonal antibody MAb32B11 against glomalin was also performed [24].
Data analysis
The dry weights of the shoots and roots were measured, and the effects of treatments on plant growth and glomalin content were assessed using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA)
Results and Discussion
Seedling growth and root: shoot ratio
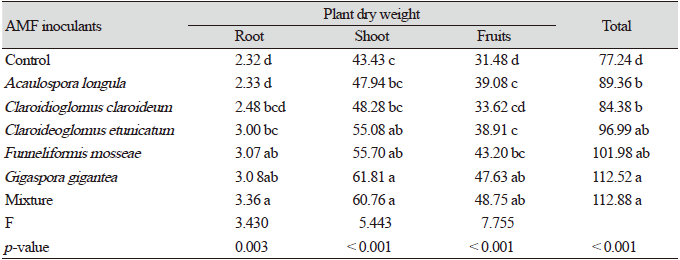
After 10 weeks of growth of pepper seedlings inoculated with AMF in the greenhouse, the dry weights of the seedlings showed significant differences (p<005, Fig. 1). The mean dry weight of the seedlings inoculated with FM and the mixture of all five species (MX) was significantly higher than that of the seedlings inoculated with other single species (p<005), suggesting that these inocula were most effective for the early growth of red pepper. In the seedlings inoculated with CE, the dry weights showed no significant increase compared to the control (p<0.05). The root to shoot ratios of CC, FM, and MX showed significant differences among the treatments (p<0.05, Fig. 1).
Growth in the field
After 10 weeks of growth in the fields, the total dry weights of the plants grown from seedlings inoculated with AMF were significantly higher than those of the control (p<0.05, Table 1). The dry weights of the roots of plants from CE-, FM-, GG-, and MX-inoculated seedlings showed a significant (p<0.05), increase compared to that of the control. Root growth was the highest in plants grown from seedlings inoculated with MX, a mixture of several AMF strains, followed by GG, FM, and CE. The dry weights of the aboveground tissue samples, except for fruits, also showed a significant difference according to the inoculum species (p<005). Plants grown from CE-, FM-, GG-, and MX-inoculated seedlings showed significant differences when compared to the control (p<0.05), and those grown from seedlings inoculated with GG and MX showed the highest dry weights of the aboveground part compared to the other treatments. The dry weights of fruits showed a significant increase (p<005) in plants grown from seedlings inoculated with CE, AL, FM, GG, and MX when compared to the control. GG- and MX-inoculated seedlings showed the highest fruit dry weight.
Glomalin content
After 10 weeks of growth in the fields, glomalin was extracted from the rhizosphere soils, and the glomalin content was measured using the Bradford assay and ELISA. The glomalin concentration measured by the Bradford assay was not significantly different among the treatments with AMF (data not shown), suggesting the influence of other proteins or organic molecules during the assay [25,26]. The ELISA results showed that glomalin content in the soil used for the growth of seedlings inoculated with AL, FM, GG, and MX was significantly higher than that in the control (Fig. 2). However, the soil used for the growth of CE- and CC-inoculated seedlings showed lower glomalin content than that of the control.
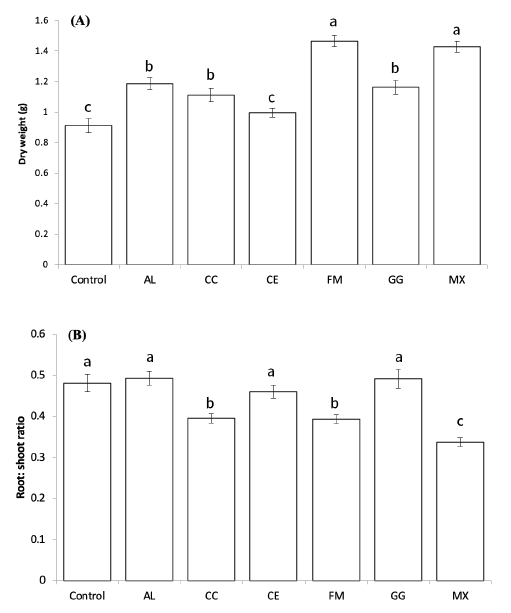
Fig. 1. Dry weights (A) and root:shoot ratio (B) ofC apsicum annuum seedlings inoculated with different fungal species after 10 weeks of growth in a greenhouse. A-C: Different letters indicate significant differences at p<0.05 (n=30) according to LSD test of one-way ANOVA. AL, Acaulospora longula; CC, Claroidioglomus claroideum; CE, Claroideoglomus etunicatum; FM, Funneliformis mosseae; GG, Gigaspora gigantea; MX, mixture.
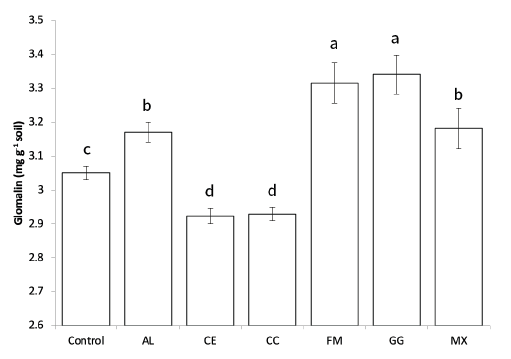
Fig. 2. Total glomalin contents (mg g-1soil) measured using ELISA (IRSP) in soils after 10 weeks of red pepper growth in fields. A-D: Different letters indicate significant differences at p<005 (n=6) according to LSD test of one-way ANOVA. AL, Acaulospora longula; CC, Claroidioglomus claroideum; CE, Claroideoglomus etunicatum; FM, Funneliformis mosseae; GG, Gigaspora gigantea; MX, mixture
In this study, AMF not only increased the growth of red pepper seedlings, but also had a positive effect on cultivated fields. Five AMF species commonly found in the soils of conventional and organic farming were used as inocula in this study, and each species was inoculated with six types of inocula, either alone or by mixing all five species. In the early growth experiments of red pepper grown in the greenhouse, the growth of pepper seedlings was significantly higher than that of the control for all species except CE. In addition, because of transplanting the seedlings to the fields and cultivating them for 10 weeks, the dry weights of the roots, stems, and fruits of the pepper plants inoculated with CE, FM, GG, and MX were significantly higher than those of the controls. Therefore, we propose that the AMF used in this study continuously affected the host plant not only in the greenhouse environment but also in the cultivated field. The positive effect of AMF on plant growth has been explained in many reports [27,28]. In addition, there was a difference in the growth of host plants depending on the AMF inoculant species treated on the same host plant [17]. These results are consistent with those of the present study. Latef et al. [29] showed that FM improved the growth performance and enhanced the salt tolerance of pepper plants. According to Castillo [30], AMF colonization increased the growth rate, foliar area, and root:shoot ratio of C. annuum under greenhouse conditions. C. claroideum, rather than a commercial inoculant, R. intraradices, positively affected the vegetative growth of chili peppers.
Glomalin content in plants inoculated with FM, AL, GG, and MX was significantly higher than that in the control. Previous studies have shown that the growth rate of mycelia in plant roots and soils differs depending on the AMF species [31]. This could be the reason why glomalin content differed depending on the AMF inoculation, suggesting that the higher the concentration of glomalin, the better the activity of the AMF [23].
Red peppers inoculated with the six different AMF inocula showed significant increase in growth both in the greenhouse and the field in this study, and in glomalin content in field soils, suggesting the positive effects of AMF inoculation on the growth of red peppers and the soil’s physical properties by increasing glomalin concentration in field soil.
Maintaining soil health and productivity is the central goal of sustainable agriculture. AMF have received attention as eco-friendly biofertilizers because they improve plant growth and help plants overcome various stressful environments; in addition, AMF improve soil fertility by producing glomalin. Despite the many beneficial roles of AMF, the commercialization of AMF as biofertilizers is still limited due to the lack of cost- effective mass production techniques and the possible impact of conservation techniques on efficiency. This study investigated the effects of AMF inocula entrapped in alginate beads and air-dried on pepper plant growth and glomalin production, and showed that AMF inoculation significantly improved the growth of pepper plants and glomalin content. The application of AMF in agriculture would contribute positively to sustainable agriculture by reducing chemical fertilizer use while increasing crop growth.