Strawberries (Fragaria x ananassa) are a high income crop in Korea. In 2022, they were ranked as the third-highest income crop among greenhouse vegetables, follow cucumbers and eggplants, they also ranked fourth in terms of cultivation area among fruit and vegetable crops [1]. In Chungnam Province, various strawberry varieties such as Seolhyang [2] and Kingsberry [3] are cultivated.
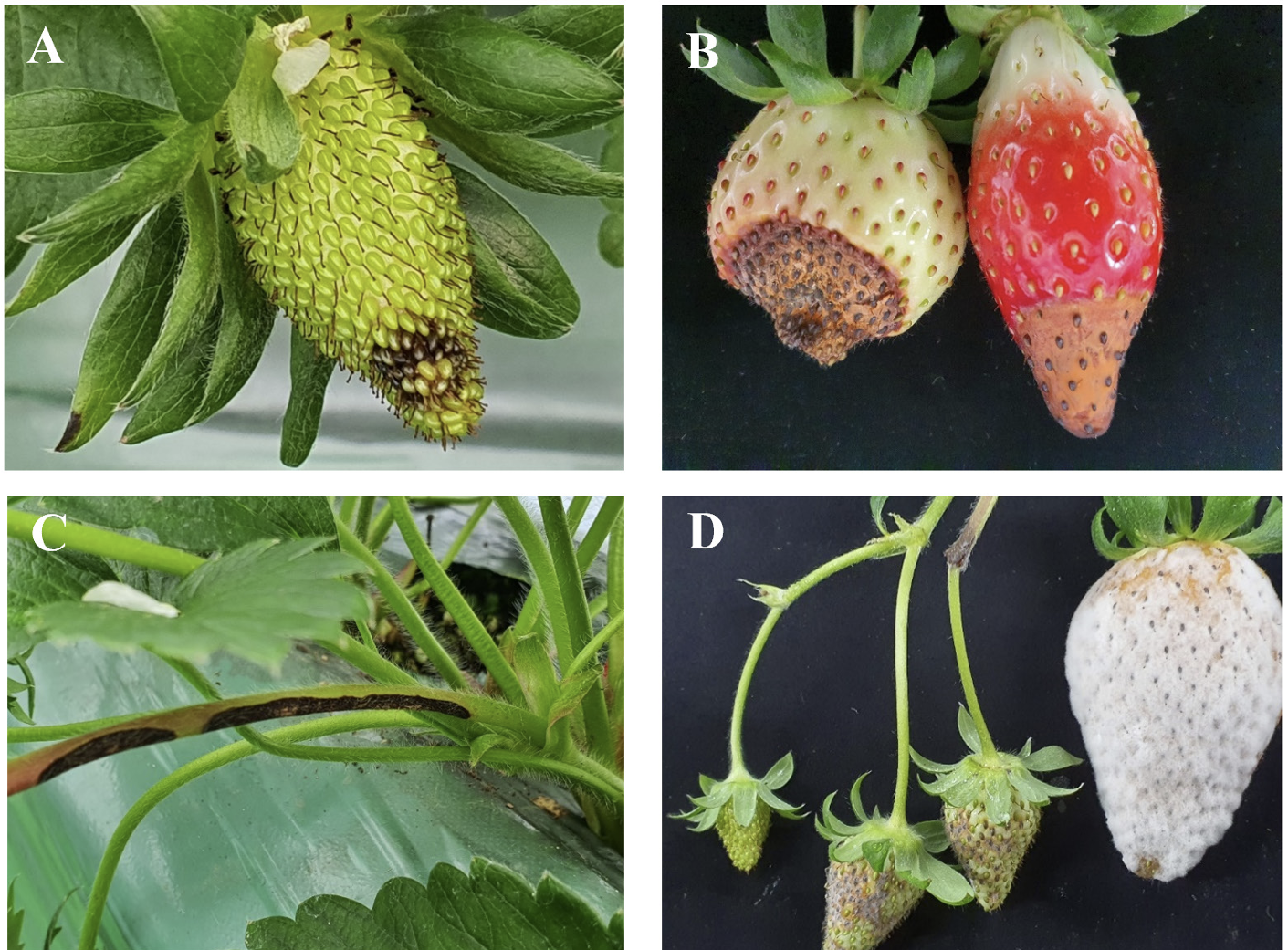
In November 2021, anthracnose symptoms were observed on strawberries (cv. Kingsberry) in Nonsan, Chungnam Province. In the early stages of anthracnose fruit rot (AFR), the symptoms include blackening of certain tissues of the strawberry (Fig. 1A), followed by the enlargement of lesions, hindering proper fruit development, and the formation of orange-colored spores within the lesions (Fig. 1B). Similar symptoms were observed on the petioles, with sunken and blackened tissues similar to those on the fruit, accompanied by the formation of an orange conidial mass (Fig. 1C).
Fig. 1
Anthracnose symptoms of Colletototrichum nymphaeae on strawberry. (A) The initial symptom of anthracnose fruit rot on the surface of strawberry fruit. (B) The exacerbated symptom on the surface of strawberry fruit. (C) The symptom of anthracnose fruit rot on the peduncle. (D) Symptoms of artificial inoculation test with an isolate.
In Korea, AFR caused by Colletotrichum acutatum (J. H. Simmonds) was first reported in 2008 [4]. However, the primary concern here is anthracnose crown rot (ACR), not AFR [5]. Initially reported as caused by C. gloeosporioides, ACR was reclassified as resulting from C. fructicola based on molecular phylogenetic analyses [6].
To identify the fungus causing the AFR, infected fruit tissues displaying symptoms were first surfacesterilized with 70% alcohol for 2 min and then rinsed three times with sterilized water. Subsequently, the surface sterilized tissues were air-dried on sterilized tissue paper, placed onto water agar, and incubated at 25℃. Following incubation, the edges of the fungal mycelia grown from the tissue were transferred to potato dextrose agar (PDA; Difco, Becton Dickinson) under aseptic conditions. Single spore isolation was performed as previously described [7], and pure cultures were stored at 4℃.
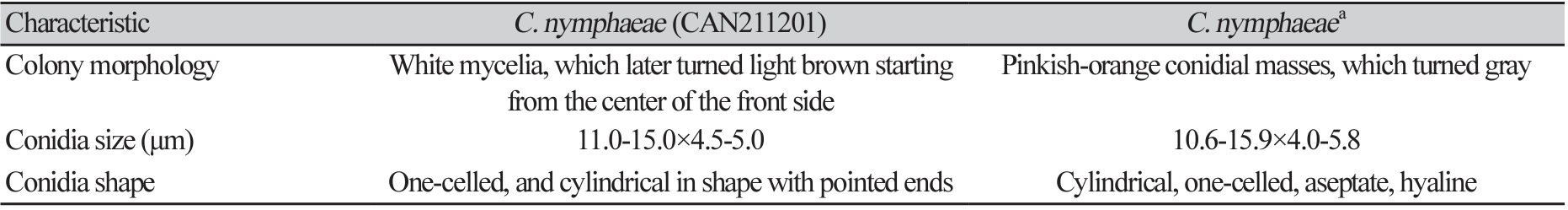
Morphological characteristics, such as the size and shape of conidia, were examined using a compound microscope (Olympus BX46, Tokyo, Japan). Based on the morphological features as reported previously [8], the isolates were identified as belonging to the C. acutatum species complex. All isolates initially formed white mycelia on PDA, which later turned light brown starting from the center of the front side (Fig. 2A and 2B). The conidia were hyaline, single-celled, and cylindrical, with pointed ends (Fig. 2C). The size range of the conidia was 11.0-15.0×4.5-5.0 µm (Table 1), with appressoria being ovate or globose and brown in color (Fig. 2D), with a size range of 7.0-11.3×5.3-7.5 µm.
Fig. 2
Cultural and morphological characteristics of Colletotrichum nymphaeae. (A and B) Front and reverse colony morphologies of CAN211201 grown on potato dextrose agar after 7 days. (C) Conidia. (D) Appressoria. Scale bar=10 µm.

Table 1
Morphological characteristics of the strain used in this study compared with a previous report on Colletotrichum nymphaeae.
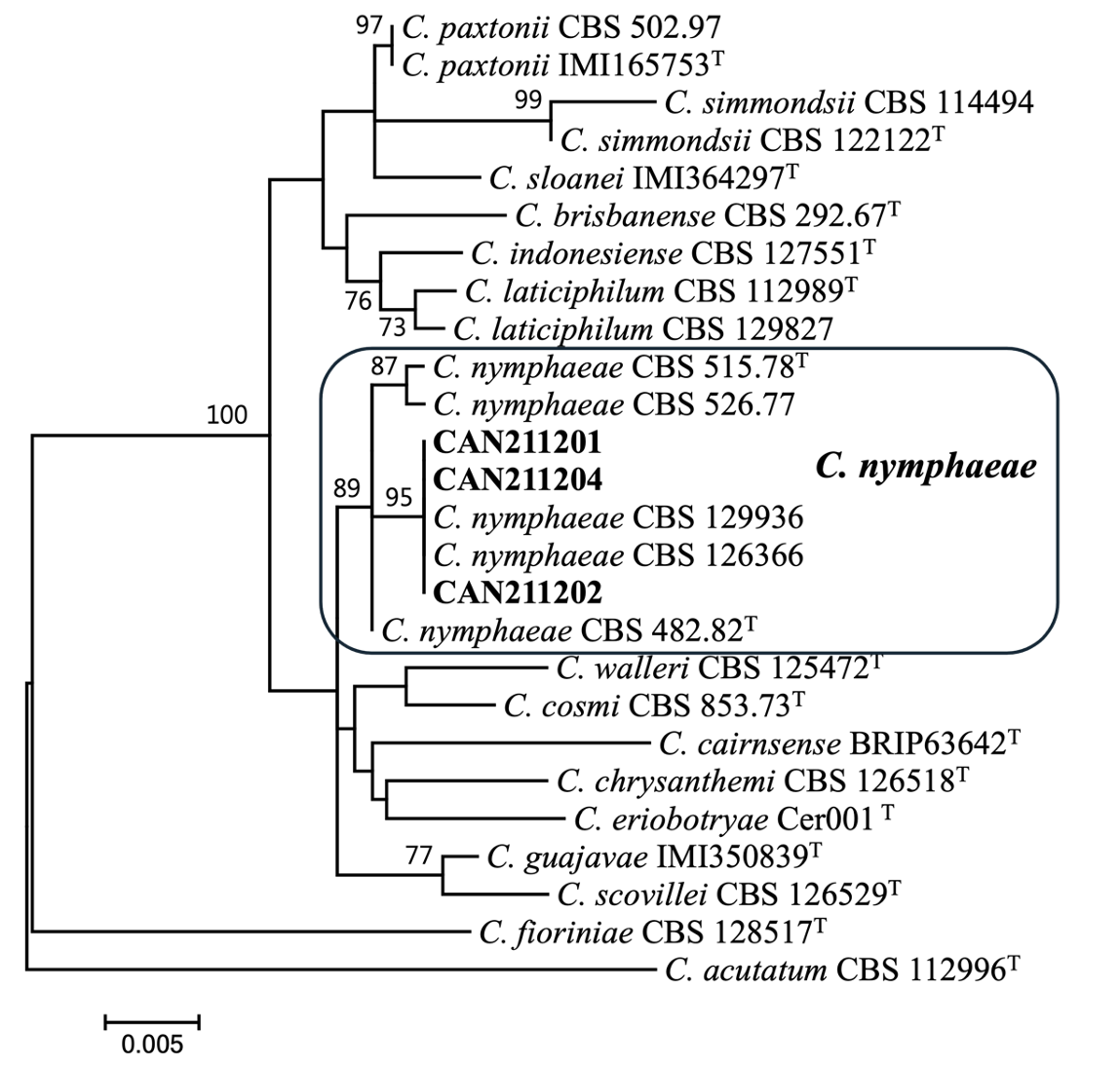
For phylogenetic classification, genomic DNA was extracted from fungal isolates grown on PDA using a modified CTAB extraction protocol [9]. The ITS, ACT, CHS-1, and GAPDH gene regions were amplified using a previously described method [10]. DNA sequencing was performed on an ABI PRISM 3730XL analyzer (Thermo Fisher Scientific, Waltham, MA, USA) at Macrogen (Seoul, Korea). Each sequence was assembled and proofread using MEGA v.7 [11]. The sequences generated in this study were deposited in GenBank (PP839268-PP839270 for ITS, PP908669-PP908671 for ACT, PP908672-PP908674 for CHS-1, PP908675-PP908677 for GAPDH). Multiple alignments were performed using MAFFT ver. 7 [12]. Maximum likelihood phylogenetic analyses were conducted using RAxML [13,14] implemented on the CIPRES web portal using the GTR+G model with 1,000 bootstrap replicates. Phylogenetic analysis revealed that three isolates formed a monophyletic group with C. nymphaeae CBS 515.78 (ex-type), CBS 526.77, CBS 129936, and CBS 126366 (bootstrap support=89%; Fig. 3).
Fig. 3
Maximum likelihood phylogenetic tree based on the concatenated datasets (ITS, ACT, CHS-1, and GAPDH) used to identify Colletotrichum strains isolated from strawberry in Korea. Bootstrap scores greater than 70 are presented at the nodes. The scale bar indicates the number of nucleotide substitutions per site and the letter T indicates ex-type strains. The strains originating from strawberry are indicated in bold.
The isolate, CAN211201, was utilized for artificial inoculation for pathogenicity testing and a suspension of 1×105 conidia/mL was sprayed onto 1 mL of each of the five fruits of the ʻKingsberry’ cultivar. Five fruits treated with sterilized water were used as controls. The inoculated fruits were kept in a moist box (100% relative humidity) at 25℃ for 5 days. During pathogenicity testing, the isolated strain exhibited typical symptoms of AFR on fruits, initially showing a black lesion. Eventually, due to high humidity, the lesion was covered with white fungi (Fig. 1D), while the controls remained symptom-free. The fungus was successfully re-isolated and identified as C. nymphaeae through morphological and phylogenetic analysis.
Morphological and phylogenetical analyses revealed that these three isolates were identified as C. nymphaeae, and pathogenicity tests indicated that the isolate showed typical symptoms of AFR.
Previously, C. acutatum, not C. nymphaeae, has been reported to cause AFR in strawberries in Korea. However, C. nymphaeae has been reported in strawberry plants in Egypt [15] and Argentina [16]. Additionally, anthracnose caused by C. nymphaeae in Korea has been reported in kiwiberries [17], Japanese plums [18], and persimmons [19]. Given these findings, it is necessary to investigate whether C. nymphaeae originated from other plants or countries. In this study, C. nymphaeae inducing AFR in strawberry fruits was confirmed based on morphological features and molecular phylogenetic analyses. Therefore, continuous monitoring should be conducted to determine if C. nymphaeae causing AFR occurs in other strawberry cultivation regions.

