INTRODUCTION
Apples (Malus domestica) are an important crop in Korea, which produced 394,428 tons of fruit and cultivated 33,789 ha in 2023, of which 59% of the cultivated regions were located in the Gyeongbuk province [1]. Fungal diseases are a primary cause of reduced fruit quality and yield in apple production [2]. For example, fungal species that cause sooty blotch and flyspeck (SBFS) colonize the epicuticular wax layer of apple fruits; this causes no physiological damage to the living cells [3]. The blemishes on apple fruits surfaces are caused by the mycelial mat and sclerotium-like body of the SBFS-causing species and can decrease their fresh market value by up to 90% [4]. In addition, these fungal species can accelerate the apples weight loss and shriveling during cold storage [5]. Therefore, SBFS-causing fungi are economically significant pathogens in apples worldwide. Globally, more than 100 species have been recorded to cause SBFS, including species from the genera Peltaster, Schizothyrium, Ramularia, and Cyphellophora [6]. The genus Peltaster was established by Sydow and Sydow (1917) based on P. hedyotidis Syd. & P. Syd. isolated from Hedyotis elmeri (Rubiaceae) in the Philippines [7]. In 1996, P. fructicola was first described as causing SBFS with a punctate mycelial type on apple fruits and the stems of brambles (Rubus spp.) in the United States of America (USA) [8,9]. Subsequently, P. cerophilus and P. gemmifer have been associated with causing SBFS on apples in Slovenia and USA, respectively [7,10]. In Korea, two species, Gloeodes pomigena and Schizothyrium pomi, have been reported as SBFS causal agents on apples [11]; however, there are no previously reported studies on the genus Peltaster species that may be associated with SBFS on apples in Korea. Therefore, apple fruits showing symptoms of SBFS were collected from apple orchards in Cheongsong-gun and Bonghwa-gun in the Gyeongbuk province of Korea in 2023. In this study, we report unrecorded pathogen that caused SBFS on apples in Korea based on its morphological characteristics and phylogenetic analysis.
MATERIAL AND METHODS
Isolation source and methods
Apple fruits (cv. Fuji) with SBFS disease symptoms were collected from apple orchards located in Cheongsong-gun (36°17′09.6″N 128°57′30.9″E) and Bonghwa-gun (36°54′09.4″N 128°58′14.5″E) in the Gyeongbuk province of South Korea between August and September 2023. The disease appeared as dark regular or irregular sclerotium-like bodies with mycelial mats on the apple surfaces. The causal agents of the SBFS were isolated from symptomatic apples according to the method of Medjedović et al. [10]. First, the apple surfaces were sterilized with 70% ethanol. Then, the presumptive causative agents of the SBFS were transferred from the apple peels to potato dextrose agar (PDA) (Difco™, Becton, Dickinson and Company [BD], Franklin Lakes, NJ, USA) and cultured at 25℃ in the dark. After 2 weeks, small black colonies were observed, and the margin of each colony was transferred to new PDA plates. The five isolated strains were designated as KNUF-23-BH01, KNUF-23-BH03, KNUF-23-CS02, KNUF-23-CS06, and KNUF-23CS12; strain KNUF-23-BH03 was selected for further morphological and cultural characterization.
Cultural and morphological characteristics
Cultural characteristics of the KNUF-23-BH03 were observed after growth on PDA and 2% water agar (WA) following the method of Williamson et al. [9]. Mycelial plugs were taken from the margin of the colony using a 4 mm cork borer, placed on the PDA or 2% WA, and then incubated at 25℃ in the dark for 3 weeks. Morphological characteristics were observed using synthetic nutrient-poor agar (SNA) and 2% WA according to a previously reported method [12]. The conidia and conidiogenous cells of the fungal specimens were observed and measured using an optical microscope (Olympus BX-50; Evident Corp., Tokyo, Japan). The diameter of the colonies was measured with vernier calipers (Mitutoyo Corp., Kawasaki, Japan).
Genomic DNA extraction and amplification
The total genomic DNA was extracted from the fungal strain KNUF-23-BH03 grown on the PDA using a HiGene™ Genomic DNA Prep Kit (Biofact Co., Ltd., Daejeon, Korea) according to the manufacturer’s protocol. For molecular identification of the strain, the internal transcribed spacer (ITS) region, nuclear large subunit (LSU) ribosomal DNA region, and mitochondrial small subunit (mtSSU) ribosomal DNA region were amplified with primer pairs ITS1F/ITS4, LROR/LR5, and mrSSU1/mrSSU3R [13- 17], respectively. The amplified PCR products were purified using ExoSAP-IT (Thermo Fisher Scientific Inc., Waltham, MA, USA) and sequenced by Solgent Co., Ltd. (Daejeon, Korea). All the obtained sequences were registered in GenBank as PP814955–PP814959 for the ITS region, PP814967-PP814971 for LSU, and PP824772PP824776 for mtSSU.
Molecular phylogenetic analysis
The phylogenetic analysis was conducted using retrieved sequences registered on the NCBI (Table 1). The ambiguous regions were deleted from the alignments and the evolutionary distance matrices for the maximum likelihood (ML) method were calculated using the Clustal X program and Tamura-Nei model [18]. The ML method was used for the construction of phylogenetic trees MEGA 11.0 software with bootstrap values based on 1,000 replications [19].
Pathogenicity test
Pathogenicity test was conducted to verify Koch’s postulates by inoculation of the KNUF-23-BH03 strain on healthy apple fruits (cv. Fuji) using a modification of the method mentioned by Johnson et al. [20]. First, the apple fruits were washed using tap water, surface sterilized with 70% ethanol, and then dried for 2 min. An inoculum suspension was prepared by homogenizing 0.05 g of mycelial fragments grown on PDA for 2 weeks with 1 mL of double-distilled water; the suspension was supplemented with 0.1% Tween 20. Then, sterilized paper discs that had been dipped in the inoculum suspension for 2 min were attached to the surface of the healthy apples. Inoculated fruits were incubated in a moist chamber at 25℃ for 3 weeks.
RESULTS AND DISCUSSION
Peltaster fructicola Eric M. Johnson, T.B. Sutton & Hodges, Mycologia 88:120 (1996) [MycoBank#434481]
The mycological characteristics and molecular phylogeny of the five strains KNUF-23-BH01, KNUF23-BH03, KNUF-23-CS02, KNUF-23-CS06, and KNUF-23-CS12 isolated in this study were similar to each other. Therefore, the cultural and morphological characteristics of the KNUF-23-BH03 were described as the representative strain in this study.
Cultural and morphological characteristics of KNUF-23-BH03
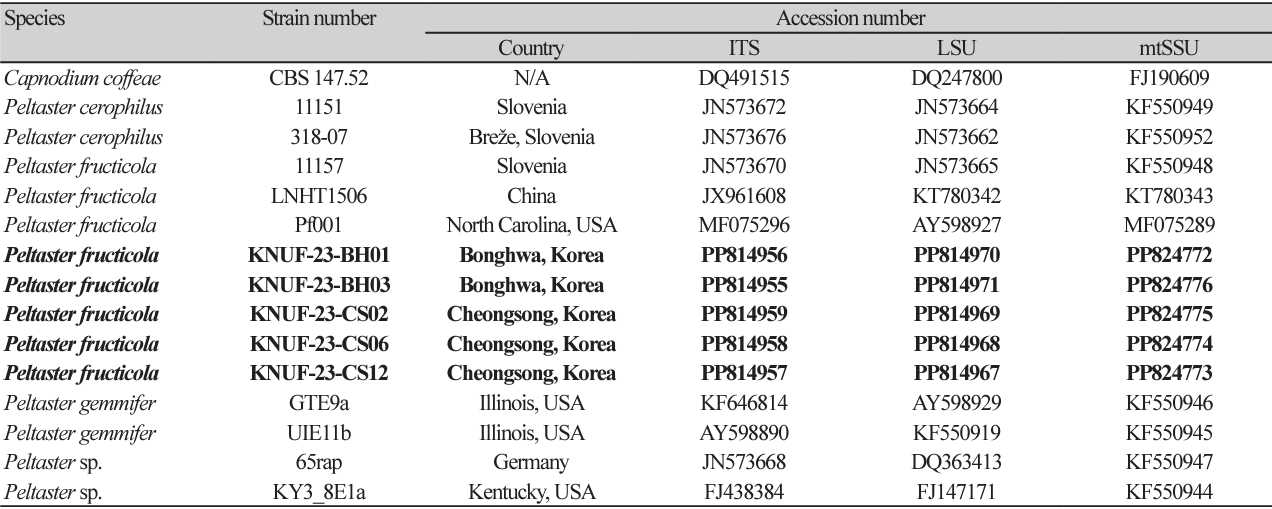
The KNUF-23-BH03 showed a punctate mycelial type on the apple peel, including a visible mycelial mat with darkly pigmented, circular, or irregular sclerotium-like bodies, 65-163 μm diameter (n=50) (Fig. 1A-C). Previously, the sclerotium-like bodies of P. fructicola LNHT1506 and Pf001 were reported to be 68-145 μm and 19-282 μm in diameter, respectively [9,12]. The colony of KNUF-23-BH03 reached 1217 mm (av.=14 mm) in diameter after incubation at 25℃ for 3 weeks on PDA. The colonies, which were slow-growing, erumpent, dense, irregular with smooth margins, wrinkled surfaces, and had no aerial mycelium; in addition, primarily yellowish conidia masses that were reverse turned dark beige to olive-gray (Fig. 1). The colony of P. fructicola LNHT1506 and Pf001 grown on PDA have been previously reported as 13-16 mm (av.=14 mm) and 11-23 mm (av.=14 mm) in diameter, respectively; P. fructicola Pf001’s colonies have been reported as 8-22 mm (av.=14 mm) in diameter when cultivated on WA [9,12].
Furthermore, conidiophores were absent in the KNUF-23-BH03 and the hyphae were septate, branched, hyaline, and turned brown in older cultures. The conidiogenous cells were monoblastic, hyaline, and straightly formed from the hypha. The conidia were abundant, hyaline, solitary, unicellular, cylindrical to ovoidal, and 3.5-7.0×1.7-3.9 μm in size on SNA (n=50). In contrast, the conidia were slightly smaller (4.06.6×1.8-3.2 μm) on WA compared with on SNA. The conidia of P. fructicola LNH1506 were previously reported to be 4.0-6.5×1.5-2.5 μm and 4.0-6.0×1.5-2.0 μm in size on SNA and WA, respectively. The conidia size of P. fructicola Pf001 was 3.2-7.1×1.1-2.4 μm on WA [9,12]. In addition, the formations of secondary conidia by microcyclic conidiation and budding were observed (Fig. 1H-J). Microcyclic conidiation induced by environmental agents has been previously observed in other SBFS-causing fungi [21,22]. In addition, the production of secondary conidia through budding can be source of spreading, that previously reported on other Peltaster species [7]. The morphological and cultural characteristics of the
Fig. 1
Peltaster fructicola KNUF-23-BH03. A-C: Punctate symptoms on apple fruit; D, E: Enlarged colony grown on potato dextrose agar and 2% water agar, respectively, for 21 days at 25℃ in the dark; F: Conidiogenous cells; G: Conidia; H, I: Microcyclic conidiation; J: Primary conidia producing secondary conidia by budding. K-M: Results of the pathogenicity test. White scale bars=1 mm, black scale bars=10 μm.
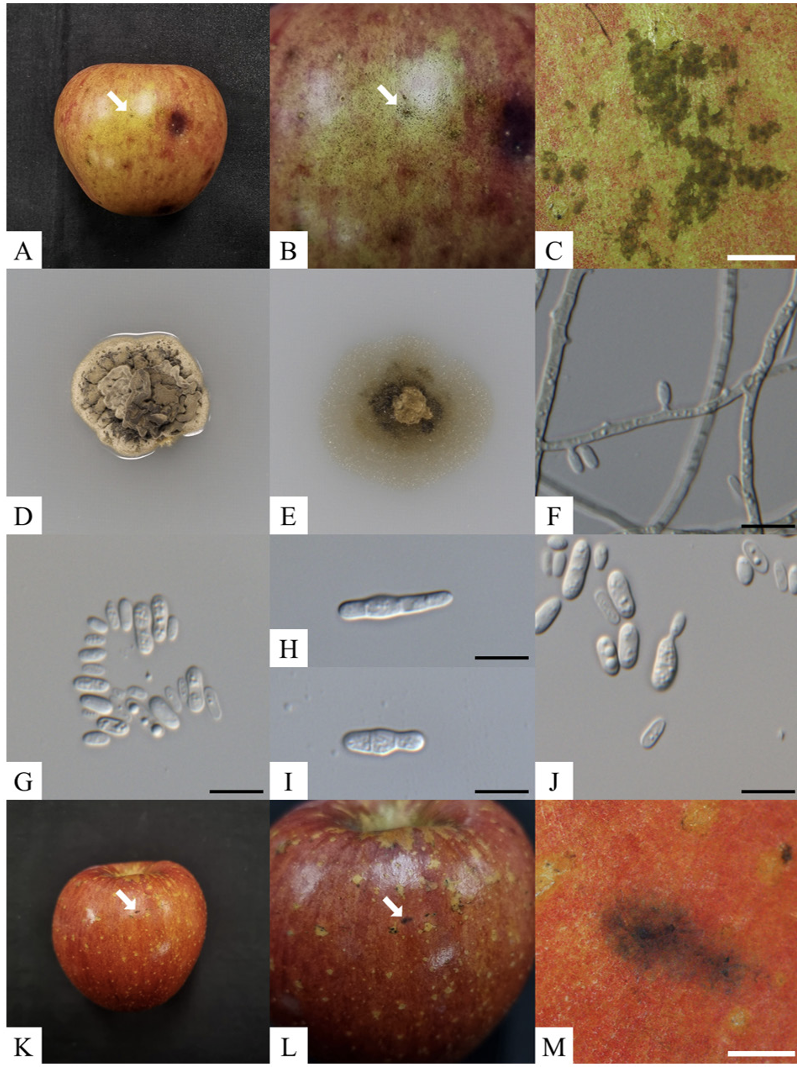
Table 2
Morphological characteristics of the strain KNUF-23-BH03 compared with those previously reported for Peltaster species
strain KNUF-23-BH03 were similar to those of previously identified P. fructicola (Table 2) [9,12].
Molecular phylogenic analysis
The partial sequences of the ITS regions (588 bp), and the LSU (810 bp) and mtSSU (781 bp) were obtained for the isolated strains. The BLAST results of the partial ITS region sequences from the five strains revealed a similarity of 98.7-100% with various P. fructicola strains including Pf001, LNHT1506, and 11157. However, strain revealed 84.7% similarities with P. cerophilus 318-07 and 11151, 87.5% similarities with P. gemmifer GTE9a and UIE11b. In the case of the partial LSU sequence, the similarity was 100% with P. fructicola Pf001, LNHT 1506, and 11157, and below 98.1% and 96.9% with P. gemmifer (GTE9a and UIE11b) and P. cerophilus (318-07 and 11151), respectively. The partial mtSSU sequence of the five isolated strains revealed 99.7-99.9% similarity with P. fructicola Pf001, LNHT1506, and 11157, and below 93.4% with P. gemmifer GTE9a and UIE11b and P. cerophilus 318-07 and 11151. Phylogenetic trees were constructed based on the ITS regions, LSU, and mtSSU sequences using the ML method (Fig. 2). The
Fig. 2
Maximum likelihood phylogenetic tree of the isolated strains based on the partial sequences of the internal transcribed spacer (ITS) regions, nuclear large subunit (LSU), and mitochondrial small subunit (mtSSU), exhibiting the relationship between Peltaster fructicola with the closest Peltaster species. Capnodium coffeae CBS 147.52 was used as an outgroup. The numbers above the branches indicate bootstrap values (>80%) obtained from 1,000 replicates. The strains isolated in this study are indicated in bold. Bar=0.050 substitutions per nucleotide position.
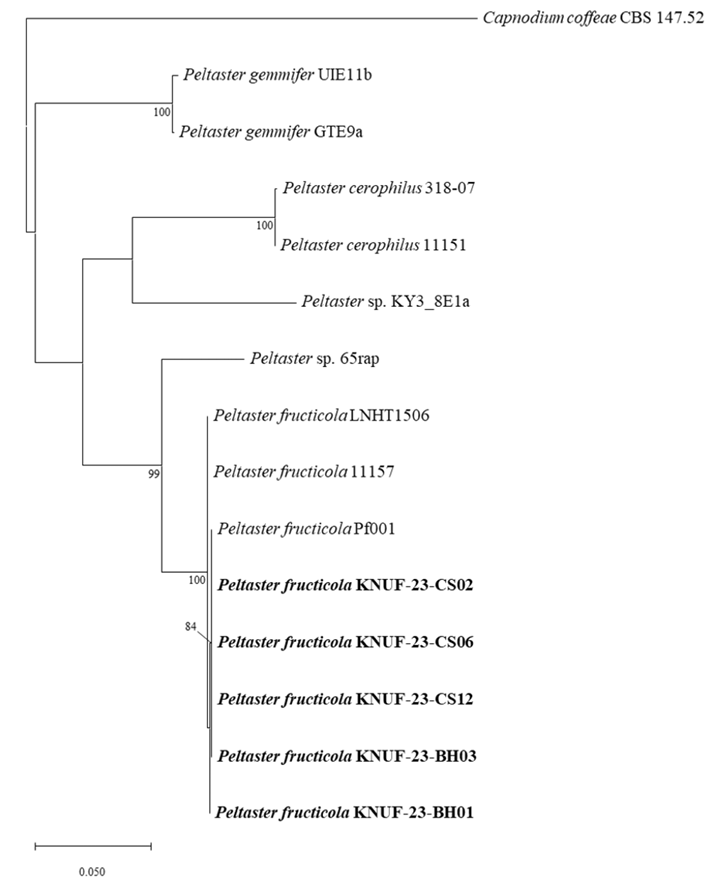
results showed that the five isolated strains were clustered with previously identified strains of P. fructicola. Therefore, the five strains isolated from the apple orchards in this study were identified as P. fructicola.
Pathogenicity test
Punctate mycelial types were observed on the apples’ surface three weeks post-inoculation, with dark, circular, or irregular sclerotium-like bodies and mycelial mats (Fig. 1K-M). The symptoms of SBFS were not observed on the control fruits. The isolated fungal agents were shown to have the same morphological and cultural characteristics as KNUF-23-BH03.
Fungi causing SBFS exhibit a series of mycelial types from only sclerotium-like bodies to mycelial mats without sclerotium-like bodies [4]; some species are distributed worldwide, while others are distributed subcontinentally or locally [6]. The P. fructicola has been previously isolated from crabapple and hawthorn fruits in China and also reported in various regions [12], such as the USA, Norway, and Turkey. In contrast, P. cerophilus and P. gemmifer have only been reported regionally [6]. In Korea, only Gloeodes pomigena and Schizothyrium pomi have been reported as causal agents of SBFS on apples; Peltaster species have not been reported previously [11]. Based on cultural, morphologic, and molecular phylogenic analyses of the strain KNUF-23-BH03 isolated from apples, were identified as P. fructicola that has been reported as the causative agent of SBFS. To our knowledge, this is the first report of the previously unreported species P. fructicola in Korea, as well as the previously unreported disease SBFS on apple fruits caused by P. fructicola in Korea.