INTRODUCTION
The phylum ascomycota morphologically diversify from unicellular yeasts to complex cup fungi. Fungal species belonging to the phylum Ascomycota are commonly known as sac fungi or ascomycetes.
Fungal species in the class Sordariomycetes show differences in morphology, growth patterns, and habitat. Emericellopsis fungi are mostly found in the upper part of the soil with high humus content [1]. Scedosporium is a ubiquitous clinically important ascomycete fungus [2]. Sce. aurantiacum strains have been recognized to cause invasive infections [3]. Bionectria is a genus in the class Sordariomycetes that grows on living plants [4]. The filamentous fungus Bionectria shows both cytotoxicity and antibacterial activity [5]. Stachybotrys is a genus of molds, hyphomycetes, and asexually reproducing filamentous fungi. Previously, fungi in this genus were found to be closely related to those in the genus Memnoniella [6, 7]. Exophiala xenobiotica is a species of black yeast that belongs to the family Herpotrichiellaceae in the phylum Ascomycota. It is morphologically close to Exo. jeanselmei, although it has less melanized conidiogenous cells. The species is a relatively common agent of cutaneous infections in humans. Natural strains of Exo. xenobiotica are often found in habitats that are rich in monoaromatic hydrocarbons and alkanes [8]. Pseudocercosporella fraxini belongs to the family Mycosphaerellaceae in the phylum Ascomycota, and Duddingtonia flagrans, belonging to the Orbiliomycetes class, is a potential biocontrol agent due to its capacity to control nematodes [9].
The purpose of this study was the morphological and molecular identification of these seven fungi that were newly discovered in Korea.
MATERIALS AND METHODS
Soil samples were collected from different field sites in Korea (Table 1) in 2016. Samples were collected at depths up to 15 cm by removing the soil surface litter. Each soil sample was air-dried and stored in a sterile polythene bag at 4°C. A conventional soil dilution technique [10] was employed to isolate the fungi, and potato dextrose agar (PDA; Difco, Detroit, MI, USA), amended with 100 µg L-1 chloramphenicol, was used for culture of the isolates. The petri plates were incubated at 26°C for 5 days, and developing colonies were transferred to fresh PDA medium to obtain pure cultures. The isolates were then transferred to PDA slants and maintained at 4°C to preserve the fungi or for further use.
Table 1. Sampled place, GPS location, GenBank accession number, and ITS rDNA sequence similarity of the studied fungal isolates
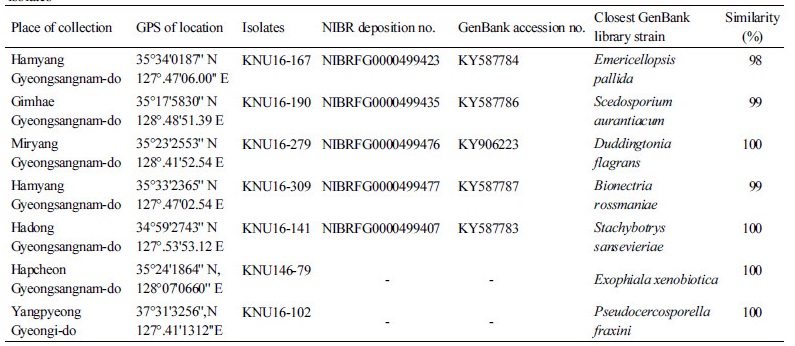
|
|
|
GPS, global positioning system; ITS, internal transcribed spacer. |
|
Morphological examination
The macro-morphological characteristics were studied using the following five different agar media: potato dextrose agar (PDA), malt extract agar (MEA), oatmeal agar (OMA), Czapek yeast extract (CYA), and yeast extract sucrose (YES). The fungal isolates were inoculated at three points on each petri plate, one with each medium, and all inoculated plates were incubated at 26°C for 7 days in darkness. Colony characteristics were recorded, and fungal materials were examined under a light microscope (Olympus BX50F-3; Olympus, Tokyo, Japan). For the micro-morphological examination, microscopic mounts of all isolates were prepared in lactic acid from colonies grown on PDA, and a drop of alcohol was added to remove air bubbles and excess conidia. Photomicrographs of the isolates were taken with an HK 3.1 CMOS digital camera (KOPTIC, Seoul, Korea) attached to an Olympus BX50F-3 microscope (Olympus). The microscopic structures of the isolates were also examined under a scanning electron microscope (LEO Model 1450VP; Carl Zeiss, Oberkochen, Germany).
Genomic DNA extraction, PCR amplification, sequencing, and phylogenetic analysis
For molecular phylogenetic identification, the isolates were grown on PDA for 1 week, and total genomic DNA was extracted using a DNeasy Plant mini kit (Qiagen, Germantown, MD, USA) following the manufacturer’s instructions. The internal transcribed spacer (ITS) region was amplified using primers ITS1 (5'-TCCGTAGGTGAACCTGCG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') [11]. The amplified PCR products were sequenced on an ABI Prism 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA). All sequence information was analyzed using the BLAST program (National Center for Biotechnology Information [NCBI], Bethesda, USA [http://www.ncbi.nlm.nih.gov/blast/]). A phylogenetic tree was constructed using the neighbor-joining method in MEGA6 [12]. The statistical confidence of the tree topology was evaluated based on a bootstrap analysis of 1,000 replicates. The sequences of all of the newly discovered fungi were deposited in the National Institute of Biological Resources (NIBR), Korea, and in GenBank (Table 1).
RESULTS AND DISCUSSION
Morphology of each of the fungal isolates
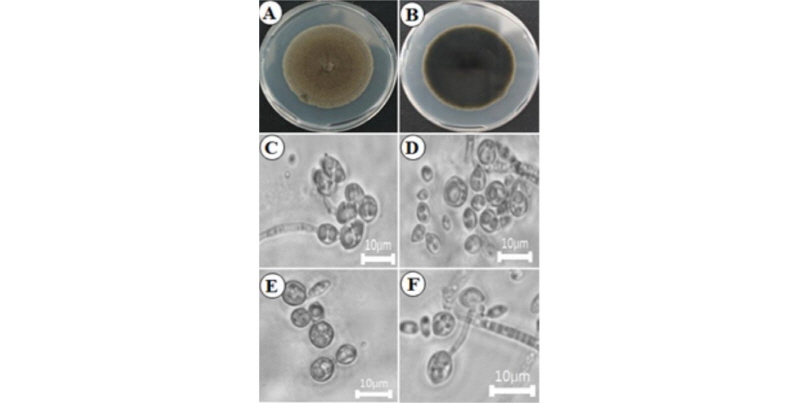
Exophiala xenobiotica (KNU16-79): Exophiala xenobiotica de Hoog, J.S. Zeng, Harrak & Deanna A. Sutton, Antonie van Leeuwenhoek 90 (3): 264 (2006) [MB#491509]
Morphological features of the isolate KNU17-79 observed by optical microscope are shown in Fig. 1. The colony grew moderately, attaining a diameter of 60~65 mm in 7 days at 26°C. (Fig. 1A, 1B). The front side of the colony was light brown, and the back side of the colony was black. Sporulation was moderate to dense (Fig. 1A, 1B). The texture of the colony was wooly.
Taxonomy of the isolate KNU16-79: Budding cells were initially abundant, pale olivaceous, ellipsoidal, and 5~6 × 2.4~3.0 µm. Hyphae were pale olivaceous to brown, 1.3~2.0 µm wide, and irregularly septate every 7~27 µm. Anastomoses were abundant. Conidiogenous cells were lemon-shaped or fusiform with flaring irregular annellated zones. Conidia were subhyaline, obovoidal, and 3.3~4.0 × 1.6~2.0 µm (Fig. 1C~1F).
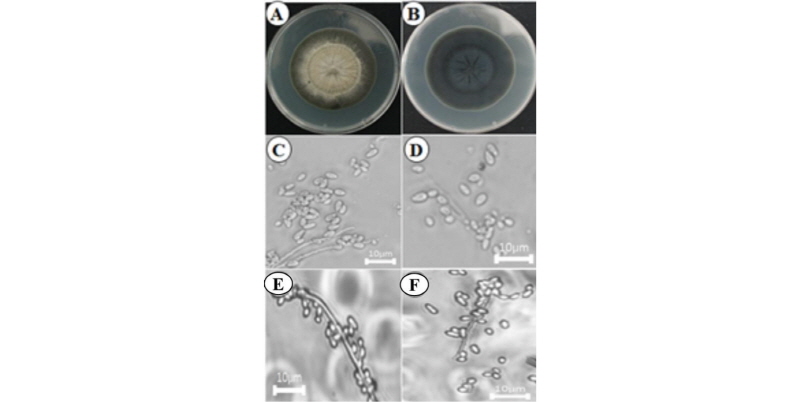
Pseudocercosporella fraxini (KNU16-102): Pseudocercosporella fraxini (Ellis & Kellerm.) U. Braun, Nova Hedwigia 58 (1–2): 212 (1994) [MB#362440]
Morphological features of the isolate KNU17-102 observed by optical microscopes are shown in Fig. 2. Colony grew moderately, attaining a diameter of 60~65 mm in 7 days at 26°C (Fig. 2A, 2B). The front side of the colony was white, and the back side of the colony was pale yellow in color. Sporulation was moderate.
Taxonomy of the isolate KNU16-102: Mycelium were internal, and hyphae were colorless, septate, branched, and 1.5~3 µm diameters, forming small- to well-developed substomatal stromata, 10~30 µm in diameter, hyaline, often somewhat erumpent, composed of somewhat swollen hyphal cells, 2~6 µm in diameter. Conidiophores fasciculate, arising from the stromata, emerging through the stomata, numerous, erect, loose to fairly dense, 10~30(~40) × 2.5~5(~7) µm, simple, hyaline, straight, subcylindric to flexuous, geniculate-sinuous, 0~1-septate, hyaline, and smooth, with conidial scars obscure. Conidia formed singly, subcylindric, vermiform, straight, curved to sinuous, 40~100(~120) × (1~)2~5(~6) µm, hyaline, smooth, apex-rounded, base more or less truncate, and unthickened (Fig. 2C~2F).
Stachybotrys sansevieriae (KNU16-141): Stachybotrys sansevieriae G.P. Agarwal & N.D. Sharma, J. Indian bot. Soc.: 78 (1974) [MB#323967]
The colony on PDA reached 25~30 mm in diameter at 26°C in 14 days. The front and back sides of the isolate were dark brown in color (Fig. 3A, 3F). Sporulation was moderate. Conidia formed in mass, with irregular and smooth surfaces. The colony on MEA attained a 28~30 mm diameter at 26°C in 14 days (Fig. 3B, 3G). The front and back sides of the colony were dark brown (Fig. 3B, 3G). Sporulation was dense and irregular in form, with a rough surface. On CYEA, the colony reached 35~40 mm in diameter at 26°C in 14 days. The front side of the colony was dark white, and the back side of the colony was light black in color (Fig. 3C, 3H). Sporulation was moderate. Spores were irregular in form and had rough surfaces. On yeast extract agar (YESA), the colony diameter reached 27~32 mm at 26°C in 14 days. The front side of the colony was white, and the back side was yellow in color (Fig. 3D, 3I). Sporulation was moderate to dense and irregular in form, and spores had rough surfaces. On OMA, the colony reached a diameter of 35~40 mm at 26°C in 14 days. The front side of the colony was dark green with white at the edges, and the back side of the colony was light yellow in color (Fig. 3E, 3J). Sporulation was moderate. Spores were irregular in form and had rough surfaces. Conidia appeared in mass.
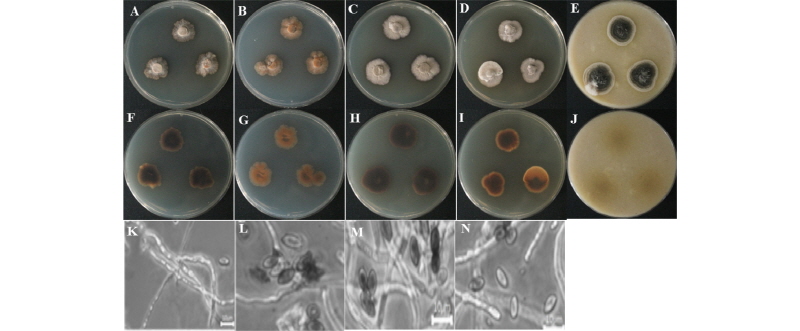
Fig. 3. Morphological characteristics of Stachybotrys sansevieriae KNU16-141 grown for 14 days on potato dextrose agar (PDA), malt extract agar (MEA), Czapek yeast extract agar (CYEA), yeast extract sucrose agar (YESA), and oatmeal agar (OMA) at 26°C. A~E, front colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; F~J, back colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; K~N, simple microscopic image of conidiophores and conidia (scale bars: K~N = 10 µm).
Taxonomy of the isolate KNU16-141: Conidia were ellipsoidal and boat-shaped, straight, truncate at the base, dark brown, smooth, and 6~9 µm in diameter (Fig. 3K~3N).
Emericellopsis pallida (KNU16-167): Emericellopsis pallida Beliakova, Mikologiya i Fitopatologiya 8: 386 (1974) [MB#8058758]
The colony on PDA reached 50~55 mm diameter at 26°C in 14 days. The front side of the isolate was pale white, and the back side was light yellow in color (Fig. 4A, 4F). Sporulation was moderate to dense. Conidia appeared in mass, were irregular in form, and had a smooth surface. The colony on MEA attained a 50~57 mm diameter at 26°C in 14 days (Fig. 4B, 4G). The front and back sides of the colony were creamy white (Fig. 4B, 4G). Sporulation was moderate to dense, irregular in form, and had a smooth surface. On CYEA, the colony reached 55~60 mm in diameter at 26°C in 14 days (Fig. 4C, 4H). The front side of the colony was white, and the back side of the colony was light yellow in color (Fig. 4C, 4H). Sporulation was moderate. Spores were irregular in form and had smooth surfaces. On YESA, the colony attained a diameter of up to 45~50 mm at 26°C in 14 days. The front side of the colony was white, and the back side was yellow in color (Fig. 4D, 4I). Sporulation was moderate to dense. Spores were irregular in form and had rough surfaces. On OMA, the colony reached a diameter of 35~40 mm at 26°C in 14 days. The front side of the colony was woolly white, and the back side of the colony was light brown in color (Fig. 4E, 4J). Sporulation was moderate. Spores were irregular in form and had rough surfaces.
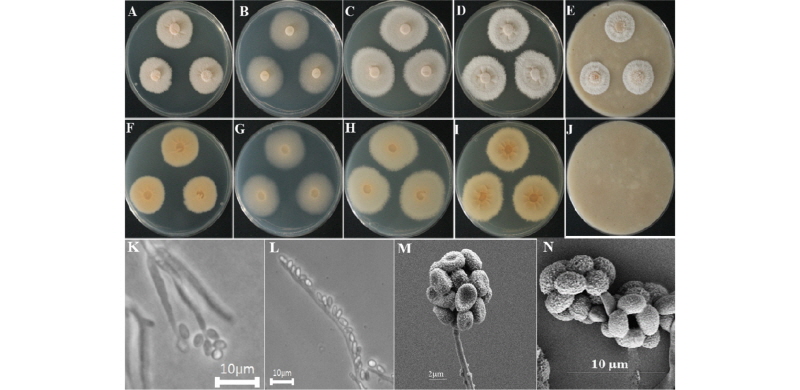
Fig. 4. Morphological characters of Emericellopsis pallida KNU16-167 grown for 14 days on potato dextrose agar (PDA), malt extract agar (MEA), Czapek yeast extract agar (CYEA), yeast extract sucrose agar (YESA), and oatmeal agar (OMA) at 26°. A~E, front colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; F~J, back colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; K, L, simple microscopic images of conidiophores; M, N, scanning electron microscopic image of conidia and conidiophores.
Taxonomy of the isolate KNU16-167: Conidial heads were radiately to loosely columnar. Stipes were 27~698 × 2.3~7.6 µm. Phialides were 4.3~7.8 × 2.0~2.4 µm in length. Conidia were globose to smooth-walled and 2.1~3.4 µm in diameter (Fig. 4K~4N).
Scedosporium aurantiacum (KNU16-190): Scedosporium aurantiacum Gilgado, Cano, Gené & Guarro, Journal of Clinical Microbiology 43 (10): 4938 (2005) [MB#357143]
The colony on PDA reached 25~30 mm in diameter at 26°C in 14 days. The front of the colony was woolly white, and back side of the isolate was yellow in color (Fig. 5A, 5F). Sporulation was moderate. Conidia appeared in mass, with an irregular form and rough surface. The colony on MEA attained a 25~30 mm diameter at 26°C in 14 days (Fig. 5B, 5G). The front and back sides of the colony were white and yellow, respectively (Fig. 5B, 5G). Sporulation was moderate to dense. Spores were irregular in form and had rough surfaces. On CYEA, the colony reached 60~65 mm in diameter at 26°C in 14 days (Fig. 5C, 5H). The front side of the colony was white, and the back side of the colony was light yellow in color (Fig. 5C, 5H). Sporulation was moderate. Spores were irregular in form and had rough surfaces. The front side of the colony had a wooly appearance. On YESA, the colony diameter reached up to 27~32 mm at 26°C in 14 days. The front side of the colony was white, and the back side was yellow in color (Fig. 5D, 5I). Sporulation was moderate to dense. Spores were irregular in form and had rough surfaces. On OMA, the colony reached a diameter of 55~60 mm at 26°C in 14 days. The front side of the colony was dark green with white edges, and the back side of the colony was light yellow in color (Fig. 5E, 5J). A cottony colony was observed. Sporulation was moderate sporulation. Spores were irregular in form and had rough surfaces. Conidia appeared in mass.
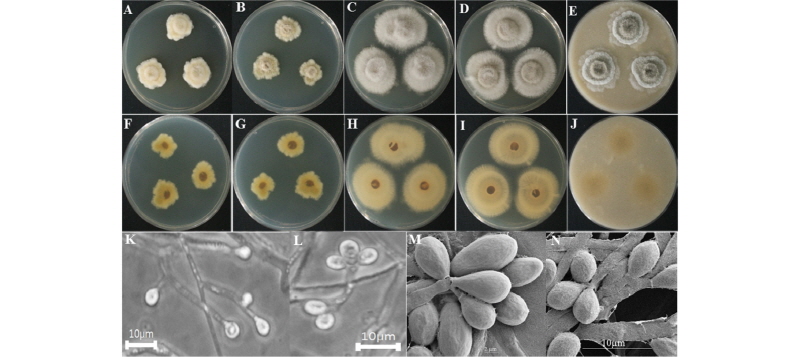
Fig. 5. Morphological characteristics of Scedosporium aurantiacum KNU16-190 grown for 14 days on potato dextrose agar (PDA), malt extract agar (MEA), Czapek yeast extract agar (CYEA), yeast extract sucrose agar (YESA), and oatmeal agar (OMA) at 26℃. A~E, front colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; F~J, back colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; K, L, simple microscopic image picture of conidiophores; M, N, scanning electron microscopic images of conidia and conidiophores.
Taxonomy of the isolate KNU16-190: Conidiogenous cells were lateral or terminal, smooth-walled, slightly flask-shaped, and 9~36 mm long of 1.3~2.2 mm wide. Conidia were smooth-walled, obovoid, or subcylindrical, with a diameter of 1.9~4.8 µm (Fig. 5K~5N).
Duddingtonia flagrans (KNU16-279): Duddingtonia flagrans Dudd.) R.C. Cooke, Transactions of the British Mycological Society 53 (2): 316 (1969) [MB#330246]
The colony on PDA reached 25~30 mm in diameter at 26°C in 14 days. The front of the colony was woolly white, and the back side of the isolate was yellow in color (Fig. 6A, 6F). Cottony growth was observed at the center and edges of the colony. Sporulation was moderate to dense. Conidia appeared in mass, and had an irregular form and rough surface. The colony on MEA attained a 65~70 mm diameter at 26°C in 14 days. The front and back sides of the colony were white (Fig. 6B, 6G). Sporulation was moderate. Spores were irregular in form and had rough surfaces. On CYEA, the colony reached 65~70 mm in diameter at 26°C in 14 days (Fig. 6C, 6H). The front side of the colony was white, and the back side of the colony was light yellow in color (Fig. 6C, 6H). Sporulation was moderate. Spores were irregular in form and had rough surfaces. The center of the colony appeared wooly. On YESA, the colony diameter reached up to 30~35 mm at 26°C in 14 days. The front and back sides of the colony were white in color (Fig. 6D, 6I). Sporulation was moderate to dense. Spores were irregular in form and had rough surfaces. On OMA, the colony reached a diameter of 55~60 mm at 26°C in 14 days. The front and back sides of the colony were white in color (Fig. 6E, 6J). Sporulation was moderate. Spores were irregular in form and had rough surfaces. Conidia appeared in mass.
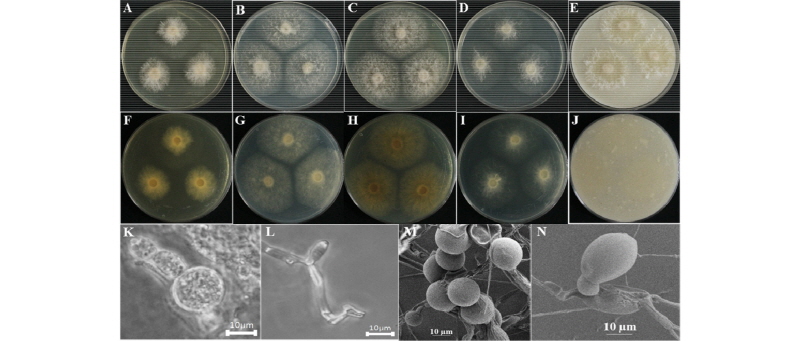
Fig. 6. Morphological characteristics of Duddingtonia flagrans KNU16-279 grown for 14 days on potato dextrose agar (PDA), malt extract agar (MEA), Czapek yeast extract agar (CYEA), yeast extract sucrose agar (YESA), and oatmeal agar (OMA) at 26℃. A~E, front colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; F~J, back colony from left to right grown on PDA, MEA, YESA, CYEA, and OMA; K, L, simple microscopic image of conidiophores; M, N, scanning electron microscopic image of conidia and conidiophores.
Taxonomy of the isolate KNU16-279: The length of conidia was 29~41 µm, their width was 13~17 µm, and the diameter of Chlamydospores was 27~32 µm. (Fig. 6K~6N).
Bionectria rossmaniae (KNU16-309): Bionectria rossmaniae, Schroers, Studies in Mycology 46: 177 (2001) [MB#485161]
The colony on PDA reached 30~35 mm in diameter at 26°C in 14 days. The front of the colony was woolly white, and the back side of the isolate was light yellow in color (Fig. 7A, 7F). Sporulation was moderate to dense. Conidia appeared in mass, were regular in form, and had rough surfaces. Colony on MEA reached 30~35 mm in diameter at 26°C in 14 days (Fig. 7B, 7G). The front and back sides of the colony were white (Fig. 7B, 7G). Sporulation was moderate. Spores had an irregular form and rough surfaces. On CYEA, the colony reached 30~35 mm of diameter at 26°C in 14 days (Fig. 7C, 7H). The front side of the colony was white, and the back side of the colony was light yellow in color (Fig. 7C, 7H). Sporulation was moderate. Spores were irregular in form and had rough surfaces. Conidia appeared in mass. On YESA, the colony diameter reached up to 30~35 mm at 26°C in 14 days. The front side of the colony was white, and the back side of the colony was light yellow in color (Fig. 7D, 7I). On OMA, the colony reached a diameter of 30~35 mm at 26°C in 14 days. The front side of the colony was white, and the back side of the colony was light yellow (Fig. 7E, 7J). Sporulation was moderate. Spores were irregular in form and had rough surfaces.
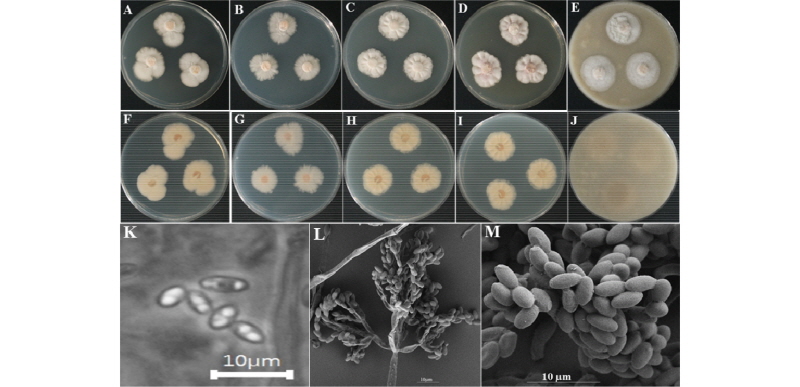
Fig. 7. Morphological characteristics of Bionectria rossmaniae KNU16-309 grown for 14 days on potato dextrose agar (PDA), malt extract agar (MEA), Czapek yeast extract agar (CYEA), yeast extract sucrose agar (YESA), and oatmeal agar (OMA) at 26℃. A~E, front colony from left to right grown on PDA, MEA, YESA, CYEA and OMA; F~J, back colony from left to right grown on PDA, MEA, YESA, CYEA and OMA; K~M, simple microscopic images of conidiophores and scanning electron microscopic images of conidia and conidiophores.
Taxonomy of the isolate KNU16-309: Ascomata appeared on white subiculum. Ascospores were fusiform and hyaline-smooth, with 7~9 gutules (Fig. 7K~7M).
Molecular phylogeny of the fungal isolates
Seven species were identified within seven different genera: Emericellopsis pallida, Scedosporium aurantiacum, Duddingtonia flagrans, Bionectria rossmaniae, Stachybotrys sansevieriae, Exophiala xenobiotica and Pseudocercosporella fraxini. A neighbor-joining tree was constructed by combining all genera with a clear sequence identity. One specimen (KNU16-141) was identified as Stachybotrys based on the ITS sequence, and a phylogram was constructed using six closely related species. This specimen formed a monophyletic clade with reference sequences of Sta. sansevieriae (bootstrap support, 100%). (Fig. 8). The isolate KNU16-167 formed a monophyletic group with two reference sequences of Eme. pallida (CBS 624.73) and KU933708 (Fig. 8). Specimen KNU16-309 grouped with two reference sequences of B. rossmaniae (bootstrap support, 99%) (Fig. 8). Isolate KNU16-190 clustered in a clade with the reference sequences of Sce. aurantiacum (bootstrap support, 99%) (Fig. 8). KNU16-279 formed a monophyletic clade with D. flagrans with 100% bootstrap support value. Isolate KNU16-79 showed 100% sequence identity with the reference isolates from NCBI GenBank (Fig. 8). KNU16-102 showed a 99% match with the reference isolate from NCBI GenBank (Fig. 8).
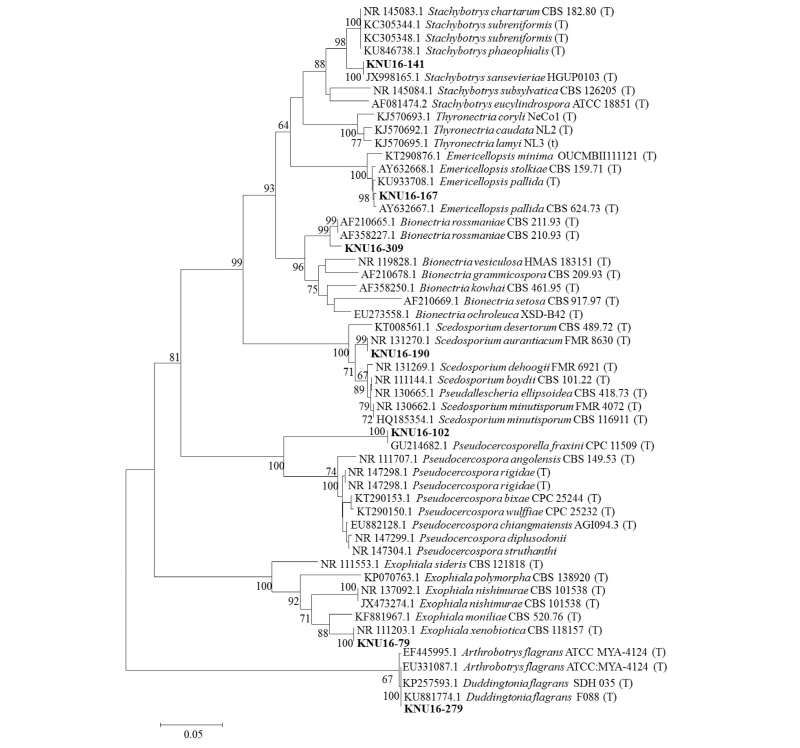
Fig. 8. Neighbor-joining phylogenetic analysis of the partial 18S-ITS1-5.8S-ITS2-28S rDNA region of KNU16-79, KNU16-102, KNU16-141, KNU16-167, KNU16-190, KNU16-279, and KNU16-309 were obtained from field soil in Korea. Numerical values (>50) on branches are the bootstrap values with 1,000 replicates. ‘T’ indicates type strains. ITS, internal transcribed spacer.
Based on these morphological and molecular characteristics, the studied fungal isolates (Exo. xenobiotica KNU16-79, P. fraxini KNU16-102, Sta. sansevieriae KNU16-141, Emericellopsis pallida KNU16-167, Sce. aurantiacum KNU16-190, D. flagrans KNU16-279, and B. rossmaniae KNU16-309) were identified. This is the first report of these fungi from soil of Korea. Further studies regarding their ecological, biological, and biotechnological importance would be worthwhile.