INTRODUCTION
Many of the fungi in the genus Gongronella are economically significant representatives of the class Zygomycetes. The cell walls of Zygomycetes are composed of chitosan [1]. Chitosan is also commercially produced from crustacean shells that are waste materials of food industries. The pin mold Gongronella butleri (Lendner) Peyronel & Dal Vesco is a well-known and important Zygomycetes fungus used in the production of chitosan. G. butlei reportedly produces the highest yield of chitosan [2]. Gongronella species have been used in biotechnological applications such as the production of antifungal proteins and enzymes [3-5]. Members of the genus Gongronella exclusively inhabit the soil [6-8] and grow relatively slowly between 25°C and 27°C [6]. Recent studies have focused on the genus Gongronella, and G. butleri, Gongronella orasabula and Gongronella koreana have been newly reported in Korea [9-11]. In this study, a fungal isolate designated KNU16-033, which was obtained from crop field soil in Korea, was identified as a member of the genus Gongronella. Based on the morphological and molecular characteristics, the isolate was identified as Gongronella guangdongensis. The isolation and identification of this strain, which is reported for the first time in Korea, is described in this report.
MATERIALS AND METHODS
Sampling and fungal isolation
Fungal isolate KNU16-033 was isolated from a soil sample collected in Daegu, Korea (N 35°53'41.6'', E 128°35'10.1''). The soil sample was collected from the ground, air-dried, and stored in a plastic bag at 4°C until analysis. A conventional dilution plating technique was applied to isolate the fungus [12]. One gram of the soil sample was suspended in 10 mL of sterile distilled water. The suspension was vortexed, diluted, and a defined volume was spread on potato dextrose agar (PDA; Difco, Detroit, MI, USA). To examine the growth rate of fungal colonies, petri dishes containing the inoculated PDA were incubated at 25°C for 3 days. Individual colonies that developed on the agar were purified by subculture on fresh PDA and incubation at 25°C until mycelium development. The pure cultures were preserved on PDA slants at 4°C.
Morphological characterization
For morphological analysis, strain KNU16-033 was grown on PDA at 25°C for 21 days. After incubation colony characteristics such as color, size, and shape were recorded. For the micro observation, samples were observed using a model BX-50 light microscope (Olympus, Tokyo, Japan).
DNA extraction, PCR and sequencing analysis
Genomic DNA of strain KNU16-033 was extracted using the HiGene Genomic DNA Prep Kit (BIOFACT, Daejeon, Korea) following the manufacturer’s instructions. To amplify the internal transcribed spacer (ITS) regions, ITS 1F/ITS 4 [13] primer pairs, and the product was purified using ExoSAP-IT (Thermo Fisher Scientific, Waltham, MA, USA) and sequenced (SolGent, Daejeon, Korea). The similarities of obtained sequences were analyzed using the BLAST in NCBI and GENETYX-WIN (ver. 3.2) program. The obtained sequences from KNU16-033 was deposited in NCBI GenBank as accession no. LC372499.
Phylogenetic analysis
To perform the phylogenetic analysis, allied Gongronella species were retrieved from GenBank. MEGA 6 software [14] was used for the alignment and the phylogenetic tree constructed by the maximum parsimony method with bootstrap analysis of 1,000 replications.
RESULTS AND DISCUSSION
Gongronella guangdongensis F. Liu, T.T. Liu & L. Cai, Cryptogamie Mycologie 36: 137 (2015)
Morphology of isolate KNU16-033
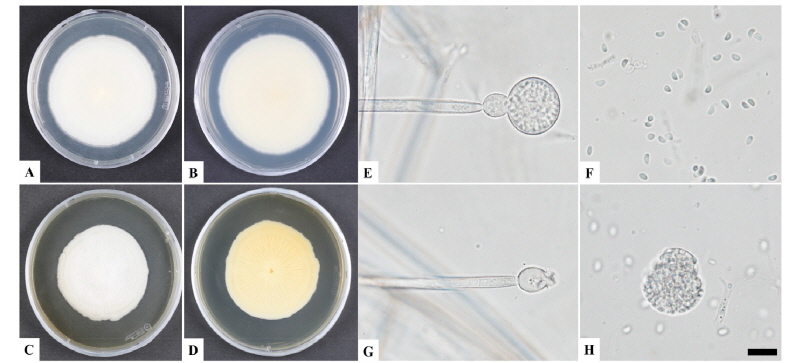
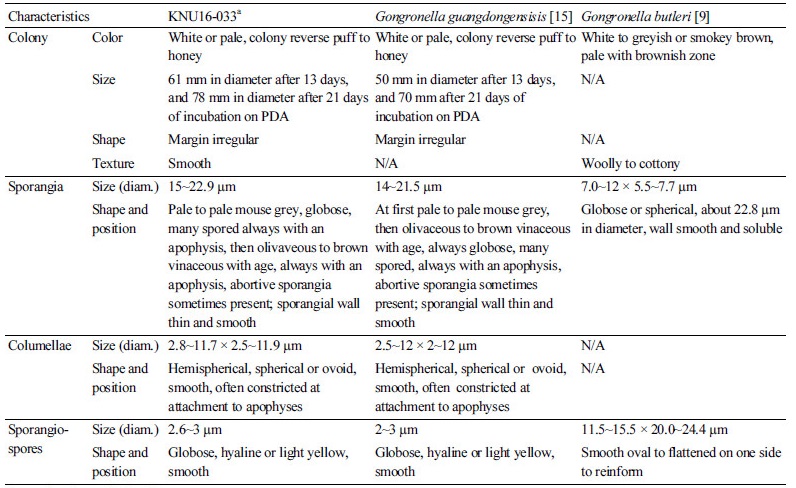
The KNU16-033 had a slow growth rate, formed white colonies and reached a diameter of 78 mm after 21 days of incubation on PDA at 25°C. Other features included erect and branched sporangiophores, globose sporangium, columellae with a globose apophysis, and one-celled sporangiospores. The morphology of the isolate was compared with previous descriptions of G. guangdongensis and G. butleri [9, 15]. These species differ morphologically based on sporangiospore characteristics. The sporangiospores of G. guangdongensis are globose and colorless or light yellow, while those G. butleri sporangiospores are hyaline, and oval to flattened on one side or almost reniform [16]. Taxonomic descriptions and microphotographs of the morphological structures of isolate KNU16-033 are presented in Table 1 and Fig. 1. Colonies having a smooth texture with a white, short, and felt-like aerial mycelium developed slowly and attained a diameter of 78 mm after 21 days of incubation on PDA (Fig. 1A, 1B). On malt extract agar (MEA), growth was slow and the colonies that formed were pale yellow to brown with an irregular margin. The colonies typically reached a diameter of 59 mm after 6 days of incubation (Fig. 2C, 2D). Columellae were hemispherical, spherical or ovoid, smooth, often constricted at the attachment to apophyses, and 2.8~11.7 × 2.5~11.9 µm in size (Fig. 1E, 1G). The sporangia were pale to pale mouse grey and always globose with many spores. An apophysis was always present and abortive sporangia were sometimes present. The sporangial wall was thin, smooth, and approximately 15~22.9 µm in diameter (Fig. 1H). Sporangiospores were globose, hyaline or light yellow, smooth, and 2.6~3 µm in size (Fig. 1F). Zygospores were not observed. These morphological characteristics of KNU16-033 were similar to those of G. guangdongensis and were distinct from those of G. butleri (Table 1).
Molecular phylogeny of isolate KNU16-033
After the sequencing analysis, 605 bp was obtained from isolate KNU16-033. As shown in Fig. 2, the KNU16-033 isolate clustered together with the G. guangdongenesis group with a 99% bootstrap value and was distinct from other Gongronella spp. in the phylogenetic tree constructed based on ITS region sequences (Fig. 2). Furthermore, it was revealed that KNU16-033 displayed 99% similarity with G. guangdongensis KC462739 that was isolated from soil. In this reason, phylogenetic analysis result support that isolate KNU16-033 was G. guangdongensis.
Gongronella species are valuable in the production of chitosan. Chitosan is an important component in the cell walls of Zygomycetes. Some Gongronella species produce a large amount of chitosan, which is economically significant in agriculture, medical, biological, and industrial applications [2, 17-19]. The chitosan-producing capability of G. guangdongensis will be the subject of future studies. G. guangdongensis is uniquely characterized by the presence of globose, hyaline or light yellow sporangiospores. The size and shape of sporangiospores are clearly distinguishing characteristics for other species of Gongronella, such as G. orasabula and G. koreana [10, 11]. To our knowledge, this is the first report of G. guangdongenesis in Korea.
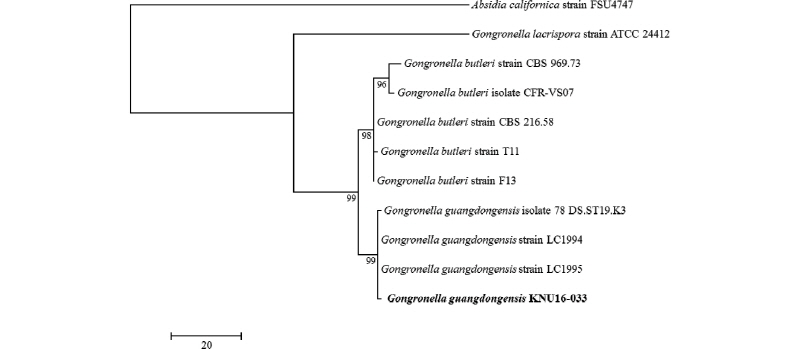
Fig. 2. One of the ten most parsimonious trees generated from maximum parsimony analysis of obtained sequences of the internal transcribed spacer rDNA region. Absidia californica strain FSU4747 was an outgroup. The KNU16-033 strain is in bold. The scale bar indicates the number of nucleotide substitutions.