INTRODUCTION
Wood decay fungi are fungal species that obtain their energy source from both dead and living wood. Thus, they play an essential role in nutrient cycling in forest ecosystems [1, 2]. Wood decay fungi are divided into white rot and brown rot based on their ability to degrade cellulose, hemicellulose, and lignin [3]. Each variety produces different kinds of enzymes and can degrade different plant materials [4, 5]. The enzymes produced by decay fungi have been utilized in industrial applications, such as in biofuel production and medicinal applications [6-8].
Most wood decay fungi belong to the order Polyporales in the phylum Basidiomycota. They have several shapes of basidiocarps including bracket-shaped, effused resupinate, and stipitate. Based on morphological characters only, species identification in Polyporales is inadequate owing to the relatively simple characteristics and morphological similarity [3]. Since the introduction of molecular methods, the internal transcribed spacer (ITS) region has been proposed as the fungal barcode gene for identification of fungi at the species level [9]. Recently, we discovered new species and unrecorded species of wood decay fungi using morphological characters and ITS sequence analysis [10, 11].
Currently, approximately 200 polyporoid fungi have been reported in Korea [12]. Since the adoption of the ABS protocol in recent years, the importance and discovery of biological resources including decay fungi have received greater attention. In 2017, the Korea National Park Service initiated a survey of macrofungi in Dadohaehaesang National Park to determine macrofungi biodiversity. During the identification of macrofungi by studying morphological features and nucleotide sequences of ITS regions, we discovered two previously unrecorded species of Cinereomyces and Steccherinum in Korea. In the present study, we provide the macro- and micro-morphological characteristics of these newly recorded species in detail.
MATERIALS AND METHODS
Samples and morphological observations
Specimens of macrofungi were collected from Dadohaehaesang National Park in Korea during 2017. Specimens were dried and deposited in the Seoul National University Fungus Collection (SFC). The specimens were initially identified based on macro- and micro- morphological characteristics [3, 13, 14]. Dried tissue was rehydrated in 3% (w/v) KOH and stained in 1% (w/v) phloxine using a Nikon 80i light microscope (Nikon, Tokyo, Japan). Basidia (30 per sample) and basidiospores (10 per sample) were measured. The quotient (Q) refers to the length/width ratio of individual basidiospores.
DNA extraction, PCR, sequencing, and phylogenetic analysis
Genomic DNA was extracted using a modified cetyltrimethylammonium bromide extraction protocol [15]. PCR reactions were performed using the primers ITS1F and ITS4B [16]. Each PCR amplification was performed in a C1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA) described by Park et al. [17]. The PCR products were purified using an Expin PCR purification kit (GeneAll, Seoul, Korea). DNA sequencing was performed at Macrogen (Seoul, Korea) using an ABI Prism 3730 Genetic Analyzer (Life Technologies, Gaithersburg, MD, USA).
DNA sequences were assembled, proofread, and edited using MEGA ver. 5.0 [18]. The resulting sequences were deposited in GenBank (accession numbers presented in Table 1). Reference sequences of Cinereomyces and Steccherinum were downloaded from GenBank and multiple alignments were performed using the default settings of MAFFT v7 [19]. Maximum likelihood phylogenetic analyses were constructed using RAxML [20], with the GTR+G model of evolution and 1,000 bootstrap replicates.
RESULTS
PCR amplification of the ITS regions from specimens yielded a single band of approximately 700 base pairs and their sequences were successfully obtained. The alignment length and number of taxa sampled for Cinereomyces and Steccherinum varied: Cinereomyces (584 bp, 28 taxa) and Steccherinum (642 bp, 31 taxa). BLAST search and phylogenetic analysis of ITS regions allowed us to identify them at the species level.
Two specimens (SFC20170619-01, SFC20170619-10) of resupinated form of polypore were clustered into a monophyletic group with Cinereomyces lindbladii (bootstrap support, 100%). The specimen showed sequence similarity of 98.2~100% to reference sequences of Cinereomyces lindbladii (Fig. 1A). The genus Cinereomyces showed a close relationship with Sebipora and Ceriporiopsis. Two Steccherinum specimens (SFC20170524-15, SFC20170620-03) formed a monophyletic group with Steccherinum bourdotii (bootstrap support, 100%) in the phylogenetic analysis of 13 Steccherinum species. The specimen showed sequence similarity of 100% to Steccherinum bourdotii (JN710584) (Fig. 1B).
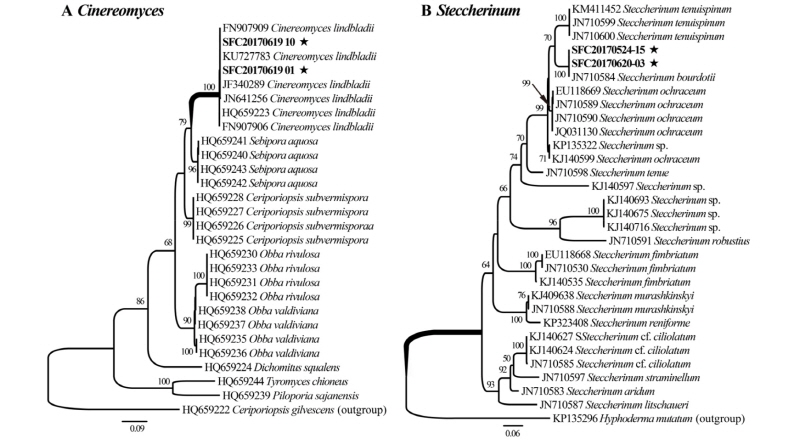
Fig. 1. Phylogenetic tree of two newly recorded species and related species based on maximum likelihood of the internal transcribed spacer. Bootstrap scores of > 70 are presented at the nodes. The scale bar indicates the number of nucleotide substitutions per site. The new records species are marked with asterisks. A, Cinereomyces lindbladii; B, Steccherinum bourdotii.
Taxonomy
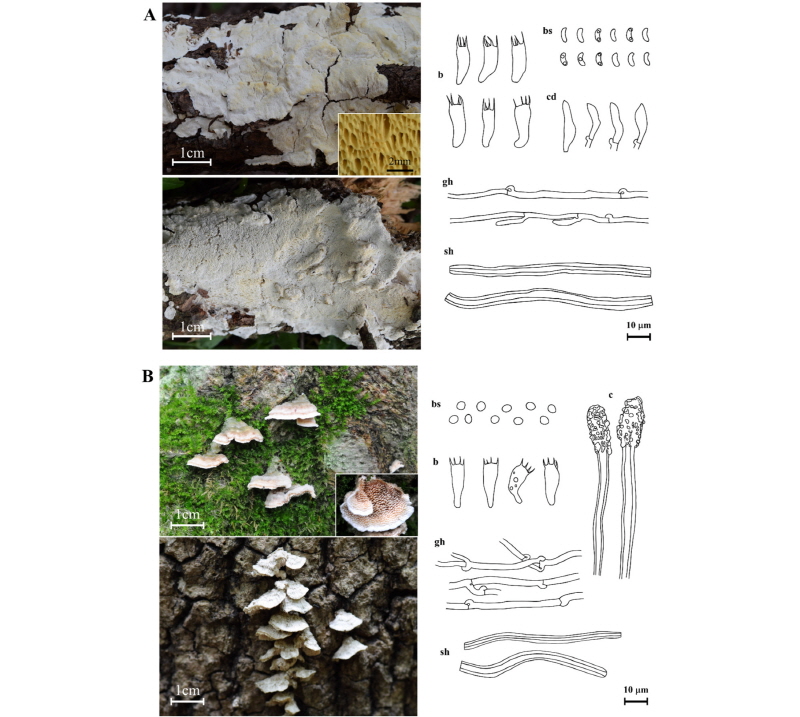
Cinereomyces lindbladii (Berk.) Jülich, Bibliotheca Mycologica 85: 400 (1982) (Fig. 2A).
Classification: Basidiomycota, Polyporales, Polyporaceae, Cinereomyces.
Basidiocarps annual, resupinate, widely effused, up to 4 mm thick, margin white; pore surface white to grayish, cracks when dry, pores circular to oblong, 2~4 per mm; context white and cottony, up to 0.5 mm thick; tube layer up to 3 mm thick, white towards the surface, lighter yellow towards the context. Hyphal system dimitic; generative hyphae with clamps, hyaline, thin-walled; skeletal hyphae straight to sinuous, thick-walled to solid, nonseptate, amyloid, dissolving in KOH. Cystidia none, but fusoid cystidioles, 12.6~14.5~ 16.0 × 2.9~3.7~4.2 µm. Basidia clavate, 4-sterigmata, 12.6~15.1~18.2 × 4.5~5.5~6.3 µm. Basidiospores allantoid to cylindrical, hyaline, thin-walled, 5.0~5.6~6.2 × 2.1~2.3~2.5 µm, Q = 2.2~2.5~2.8.
Specimen examined: Korea, Jellanam-do, Wando-gun, Cheongsando, N 34°09'35.28'' E 126°53'21.89'', on trunk of a dead tree (Pinus thunbergii), June 19, 2017, J. Y. Park, N. K. Kim, SFC20170619-01 (GenBank accession no. MG574292); Korea, Jellanam-do, Wando-gun, Cheongsando, N 34°10'15.83'' E 126°53'05.44'', on trunk of a dead tree (P. thunbergii), June 19, 2017, J. Y. Park, N. K. Kim, SFC20170619-10 (GenBank accession no. MG574293).
Remark: Cinereomyces lindbladii is morphologically similar to Cinereomyces dilutabilis. However, Cinereomyces lindbladii has larger pore basidiomes than Cinereomyces dilutabilis (6~7 per mm) [21]. This species is easily confused with Schizopora flavipora due to similar morphology and substrata. However, it can be distinguished based on the shape of the hymenophoral pore and basidiospores. Schizopora flavipora has a daedaleoid or irpicoid form of hymenophoral pore and ellipsoid basidiospore [14]. This is the first report of the genus Cinereomyces in Korea.
Steccherinum bourdotii Saliba & A. David, Cryptogamie Mycologie 9 (2): 100 (1988) (Fig. 2B).
Classification: Basidiomycota, Polyporales, Meruliaceae, Steccherinum.
Basidiocarps annual, sessile, effused-reflexed, pileate, pilei often in imbricate clusters, conchiform, up to 15 mm wide; upper surface multizonate, margin white, white fimbrillate, hymenium surface odontioid, whitish-pink, margin white. Spines conic, 2~3 mm long, 3~4 per mm. Hyphal system dimitic; generative hyphae with clamp connections, thin-walled; skeletal hyphae straight to sinuous, thick-walled, nonseptate. Cystidia numerous in the aculei, encrusted in the obtuse apex, 50.8~70.2~110.2 × 6.3~6.9~7.2 µm. Basidia subclavate, slightly sinuous, with a basal clamp, 4-sterigmata and a basal clamp, 12.0~15.2~18.9 × 5.2~5.9~6.7 µm. Basidiospores subglobose, smooth, thin-walled, 3.8~4.1~4.7 × 3.5~3.8~ 4.3 µm, Q = 1.0~1.1~1.1.
Specimen examined: Korea, Jellanam-do, Goheung-gun, Mt. Paryeongsan, N 34°38'01.20'' E 127°25'05.85'', on trunk of a dead tree (Betula sp.), May 24, 2017, J. Y. Park, N. K. Kim, SFC20170524-15 (GenBank accession no. MG574295); Korea, Jellanam-do, Goheung-gun, Mt. Paryeongsan, N 34°37'19.09'' E 127°26'15.46'', on trunk of a dead tree (Fraxinus rhynchophylla), June 20, 2017, J. Y. Park, N. K. Kim, SFC20170620-03 (GenBank accession No. MG574294).
Remark: Steccherinum bourdotii is morphologically and phylogenetically similar to Steccherinum ochraceum and Steccherinum tenuispinum. However, Steccherinum bourdotii can be distinguished from the other species by spore shape and spore size; Steccherinum bourdotii is characterized by subglobose basidiospores, whereas Steccherinum ochraceum has oval basidiospores [22]. Steccherinum bourdotii differs from Steccherinum tenuispinum which has smaller and broadly ellipsoidal basidiospores (2.8~3.9 × 2.4~2.8 µm) [23].
DISCUSSION
In the present study, we accurately identified two species (from two different genera) of wood decay fungi from Dadohaehaesang National Park based on a combination of morpho-logical observations and molecular analysis of ITS sequences. The molecular approach was conducted in two steps: 1) BLAST searches and 2) phylogenetic analysis. BLAST searches using ITS sequences are sometimes unreliable because incorrectly identified sequences in GenBank are common (approximately 20%) [24]. Therefore, phylogenetic analysis is a critical step to accurately identify specimens to the species level [25]. Through this two-step molecular approach combined with morphological observations, we added two previously unrecorded species of the Polyporales to the records in Korea.
Cinereomyces (Basidiomycota, Polyporales, Polyporaceae) is a genus of white rot fungi and is characterized by resupinate, polyporoid hymenophore, and acyanophilous and amyloid skeletal hyphae, and dissolves in KOH [21, 26]. Two species have been described in the genus Cinereomyces: Cinereomyces dilutabilis and Cinereomyces lindbladii (Index Fungorum, http://www.indexfungorum.org). Cinereomyces lindbladii is the type species of this genus. Diplomitoporus dilutabilis was transferred to genus Cinereomyces due to similar skeletal hyphae [21]. However, Cinereomyces lindbladii is easily distinguished from Cinereomyces dilutabilis by large-pored basidiomes and narrow spores. On the basis of ITS sequence analysis and morphological features, two specimens (SFC20170619-01, SFC20170619-10) were identified as Cinereomyces lindbladii, which is a previously unrecorded species in Korea.
Steccherinum (Basidiomycota, Polyporales, Meruliaceae) is a genus of white rot fungi and is characterized by odontioid to hydnoid hymenophore, cyanophilous skeletal hyphae, encrusted pseudocystidia, and smooth thin-walled inamyloid basidiospores [13, 27]. Steccherinum include approximately 60 species (Index Fungorum, http://www.index-fungorum.org). Among these, seven species have previously been reported from Korea [12]. Steccherinum bourdotii is very closely related to Steccherinum ochraceum based on morphological features. However, the two species can be distinguished from Steccherinum robustius by spore shape. Steccherinum bourdotii was reported to be a recorded species in Korean in the phylogenetic study of the genus Steccherinum; however, there is no macro- and micro-morphological descriptions for Steccherinum bourdotii [28]. By combining ITS sequence analysis and morphological features, two specimens (SFC20170524-15, SFC20170620-03) were identified as Steccherinum bourdotii. We provide macro- and micro-morphological characteristics of Steccherinum bourdotii in the present study.
Dadohaehaesang National Park is the largest national park located in the southwestern sea in Korea and consists of approximately 400 islands. Many indigenous plant species including evergreen broad-leaved trees grow in Dadohaehaesang National Park [29]. Several studies have been undertaken on the diversity of plants [30] and insects [31] in Dadohaehaesang National Park; however, there is no systematic study of fungi. Wood decay fungi play important roles as decomposers, symbionts, and pathogens in forest ecosystems [32]. Therefore, basic surveys of the fungal diversity in Dadohaehaesang National Park provide valuable information for management and conservation in this area.