Definition of endophytic fungi
Endophytes are an important field of study that has recently been undergoing rapid development, ranging from the identification to community structure, ecological role, and secondary metabolites of the endophytic fungi [1-3]. In particular, there are approximately 270,000 vascular plants [4], but the total number of endophytic fungal species is expected to be approximately 1.5 million [5], although estimates range from 100,000 [6] to 9.9 million [7]. Therefore, the diversity of fungal species may be much higher than that of plants. Thus, studies have focused on endophytic fungi that are symbiotic with plants [8] to find clues for the high biodiversity of the fungi [9].
In 1866, de Bary first recognized the endophytic fungi and assumed them to be a parasite inhabiting the reproductive organs of the plant [10]. From then onwards, the definition of endophytes was gradually elaborated on. Carroll [11], Petrini [12], and Wilson [13] regarded the endophytes as asymptomatic organisms living in healthy twigs and leaves of a plant. Therefore, endophytic fungi are present within various plant tissues from the leaves to the roots, with no apparent disease in the host plants, and contribute to maintain the balance among the host plant, the endophytes, and their environments for at least part of their life cycle.
Distribution and dispersal of endophytic fungi
The distribution pattern of the host plants and their habitats influence the ecological niche of the endophytic fungi and are essential factors for endophytic fungi biodiversity [14]. Using the currently available molecular tools, it is difficult to accurately estimate the fungal distribution and its diversity [7]; however, in general, it was known that fungal diversity is higher in tropical or subtropical regions than in temperate or arctic regions [15]. Moreover, certain endophytes are found throughout the world, while others are locally distributed [16]. Endophytic fungi have a species-specific symbiotic relationship with specific host plants, but these relationships vary widely [17]. Some endophytic fungi can occupy a specific organ of the host plant such as leaves, petiole, or twigs but other taxa invade the whole plant [9].
As plant distribution is affected mainly by climate condition, the presence of endophytic fungi is thereby determined based on the distribution of plant species, constituents such as reproductive and vegetative organs, and abiotic factors [18]. The dispersion of endophytic fungi can be attributed to two mechanisms, horizontal and vertical transmission [19]. In the former, sexual and non-sexual spores spread randomly by abiotic factors including air and water, but also by biotic factors such as insects. Fungal spores can spread over 2,000 km away, by the air current [20]. Thus, it is not possible to accurately predict the range and direction of dispersion of endophytic fungi. The vertical transmission occurs by the dispersal of host plant seeds that contain the fungal mycelium [21]. Several studies have reported that endophytic fungi can infect plant seeds, such as Festuca arundinacea Schreb, and Lolium spp., and are dispersed along with them [22]. This mechanism can provide the same environment as that for the ancestor to the next generation of endophytic fungi, but also a more consistent endophytic fungal composition to the plant; thus, the specific host plant-endophytic fungi relationships can be established, reproduced, and dispersed in nature [21]. Finally, according to these dispersion mechanisms, the fungi are distributed within the host plant and show geographical patterns. However, the distribution pattern of these endophytic fungi can be understood only in relation to the range of distribution of the host plant.
Evolution of endophytic fungi
Fossil records of fungi-like organisms go back to the Precambrian era. Since the early Devonian (408 ~ 360 Myr) fossil records of fungi have been found in the inner tissue of plants, it was believed that they have a longer evolutionary history [23, 24]. The fossil treasure of Rhynie Chert provides evidence that fungi and plants had a symbiotic relationship in the early stages of evolution. Currently, the evolutionary processes that led to the symbiotic mechanism among them are not fully understood, but few hypotheses have been suggested.
Most endophytic fungi have one of two biological forms that correspond to two different types of host plants, herbaceous and woody plants. This is the main criterion to categorize the evolutionary patterns of endophytic fungi into the two types, which are correlated with the duration of life of the host plant. Plants interact with both biotic and abiotic factors. Among herbaceous plants, the host plants respond positively to stimulation such as plant feeding and infection because they have shorter or similar length of life cycles compared to those of insects or pathogens. This interaction can be explained based on the red queen hypothesis [25]. According to this hypothesis, the adaptive properties of living organisms can influence new genotypes of several herbaceous plants that are under the influence of other organisms that are ecologically related to one another. Hence, herbaceous plants can gain adaptive properties such as increased resistance to pathogens or herbivores during the same period of the life cycle. These interaction processes can draw off different types and groups of endophytic fungi in nature [21]. In the case of woody plants, which have a more extended lifespan compared to pathogens and herbivores, the transmission of genetic material to the next generation of woody plants could be relatively slow. As a result, woody plants cannot develop a host plant-endophytic fungal symbionts during the same period of the life cycle as herbaceous plants. Therefore, woody plants can acquire adaptive characters by an indirect mechanism such as symbiosis with fungi. In particular, endophytic fungi can produce secondary metabolites that effectively impart resistance to herbivores and pathogens. The production of secondary metabolites affects plans, resulting in the plant obtaining adaptive properties from fungi by horizontal gene flow. Endophytic fungi originate from pathogens or saprophytic fungi because of evolutionary interactions [19, 26]; they interact with each other to exchange genes. Thus, the genotype of the host plant species and the genotype of the fungi may be the source of differentiation in the ancestry of endophytic fungi. After all, endophytic fungi were regarded as a polyphyletic group in the early evolutionary period. This also means that herbaceous plants and woody plants may have different lineages of endophytic fungi. Thus, morphology, gene, and secondary metabolite analysis of endophytic fungi will help shed light on their evolutionary process.
Endophytic fungi of conifers in Korea
Conifers comprise 600 to 800 taxa worldwide, of which 355 taxa are in need of conservation activities; however, 200 taxa are on the verge of extinction. In Korea, there are 30 to 60 conifer taxa, including cultivated species such as Pinus rigida, but also 3~6 taxa of endemic coniferous species, including Abies koreana [27-29].
Forest occupies 67% of the Korean landscape, which is approximately 6.6 million hectares consisting of approximately 2.7 million hectares (42%) of coniferous forest, 1.8 million hectares (29%) of mixed forest with conifers and broadleaf trees, and approximately 1.7 million hectares (26%) of broadleaf forest [30]. As climate change and deforestation occur worldwide, coniferous trees are at risk, particularly over sub-alpine regions where conifers are widely distributed but suffer severe blows. When the conifers decrease in number, there is a possibility that the symbiotic fungi also disappear; however, not enough attention has been paid to endophytic fungi that share the history of co-evolution with conifers. Because some endophytic fungi have abilities such as drought tolerance and produce protective materials, these fungi can be regarded as aids to nature to support the life of coniferous trees. Hence, many researchers are currently focusing on addressing this problem.
Indigenous species in Korea have most likely adapted to the environment of the Korean Peninsula. A. koreana is a typical endemic species and is mainly distributed on Jeju Island, Korea [31, 32]. The island was formed by volcanic activity between the Tertiary and Quaternary period of the Cenozoic era when sea level fluctuated because of the ice ages and the interglacial period [33, 34]. In other words, the sea level declined during the glacial period, when Jeju Island was connected to the continent and the Japanese islands, but rose in the interglacial period, after which, Jeju Island was isolated. At this time, during the glacial period, the northern plants could spread to the south, whereas in the interglacial period, the northern plants could move to the north or alpine regions. Therefore, the current distribution of the Korean firs of Jeju Island is a paleogeographical clue provided by repeated climate changes and geographical features [35]. This unique environment that the Korean fir was subjected to, may have resulted in endophytic fungi having a unique evolutionary relationship with the trees. For unknown reasons, the Korean fir forests have currently declined; hence, urgent investigation is required to elucidate the reason for this decline.
Although conifers of Korea comprise approximately 30~60 taxa, studies on the biodiversity of coniferous endophytic fungi in Korea are still limited to a few species; Chamaecyparis obtusa, Cryptomeria japonica, Juniperus rigida, Larix kaempferi, Pinus densiflora, P. koraiensis, P. rigida, P. thunbergii, Thuja koraiensis, and Torreya nucifera (Table 1) [36-40]. Because previous studies were mainly performed for low altitude mountains [38-41], more detailed investigation is required to include various host plants from high altitude mountains and coniferous trees over the subalpine regions.
|
Table 1. List of the endophytic fungi of gymnosperms in Korea 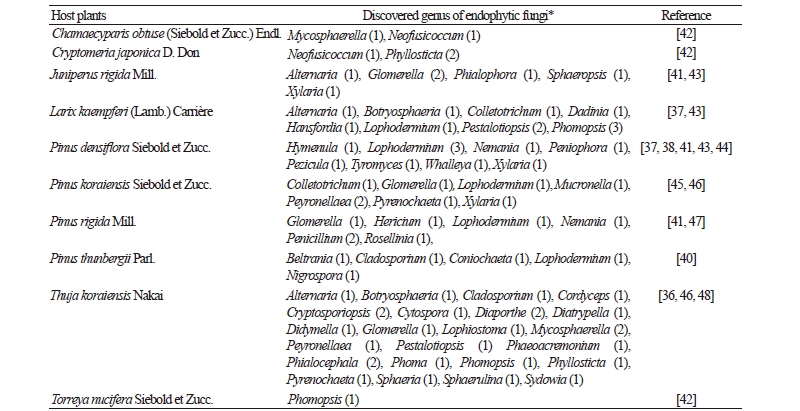
|
|
|
* The number of the endophytic species of each genus. |
|
Biodiversity of endophytic fungi is shown to have significant differences depending on the host plant. Lophodermium spp. are the dominant endophytic fungi in conifers such as P. densiflora and P. rigida, in approximately 57~79% of cases. In the case of P. thunbergii, Beltrania sp. is dominant in about 58% of this species. Phyllosticta spinarum is the dominant species in T. koraiensis, at approximately 48~65%; however, L. kaempferi and J. rigida showed no trend of dominant endophytic fungi [36-41].
Biodiversity of endophytic fungi of Pinus spp. from various geographic areas has been investigated, but those dominant in L. kaempferi and J. rigida were reported in only one or two areas; therefore, further studies need to show the detailed distribution of endophytic fungi biodiversity [37, 39, 41].
Until now, endophytic fungi of conifers in Korea were divided into 6 classes, 21 orders, 34 families, 52 genera, and 80 taxa. Based on extensive examination of literature, we believe that constant monitoring of endophytic fungi is better than a brief unrecorded species survey. This method will enable us to understand dominant or characteristic species of endophytic fungi community. The data shown here are a beginning toward elucidating the biodiversity of endophytic fungi. Further efforts are required to understand the co-evolution and ecological function of endophytic fungi.
Furthermore, it is necessary to investigate the biodiversity of endophytic fungi to better understand the distribution of all host plants, at least in Korea; this will help clarify the history of co-evolution of endophytic fungi and host plants. Current research on the biodiversity of endophytic fungi depends on traditional methods such as isolation and identification, so we cannot confirm the results of biodiversity and the dominant species of each host plant. Therefore, we will endeavor to develop new and practical tools for understanding the biodiversity of endophytic fungi in host plants.
CONCLUSION
Research on the biodiversity of endophytic fungi is at initial stages. Little is known of the characteristics, distribution, and evolutionary mechanisms of endophytic fungi. In particular, endophytic fungi that share a history of co-evolution with coniferous trees have their own ecological niche; therefore, we will need to study coniferous trees and their endophytic fungi simultaneously in terms of climate change and deforestation. In addition to the recent approaches of study using both macroscopic and microscopic tools, elucidation of biodiversity based on ecological principles must be the primary goal. Scientific findings on endophytic fungi should be well balanced from different perspectives and should enable us to have deeper insights into their ecology and evolution.

