Introduction
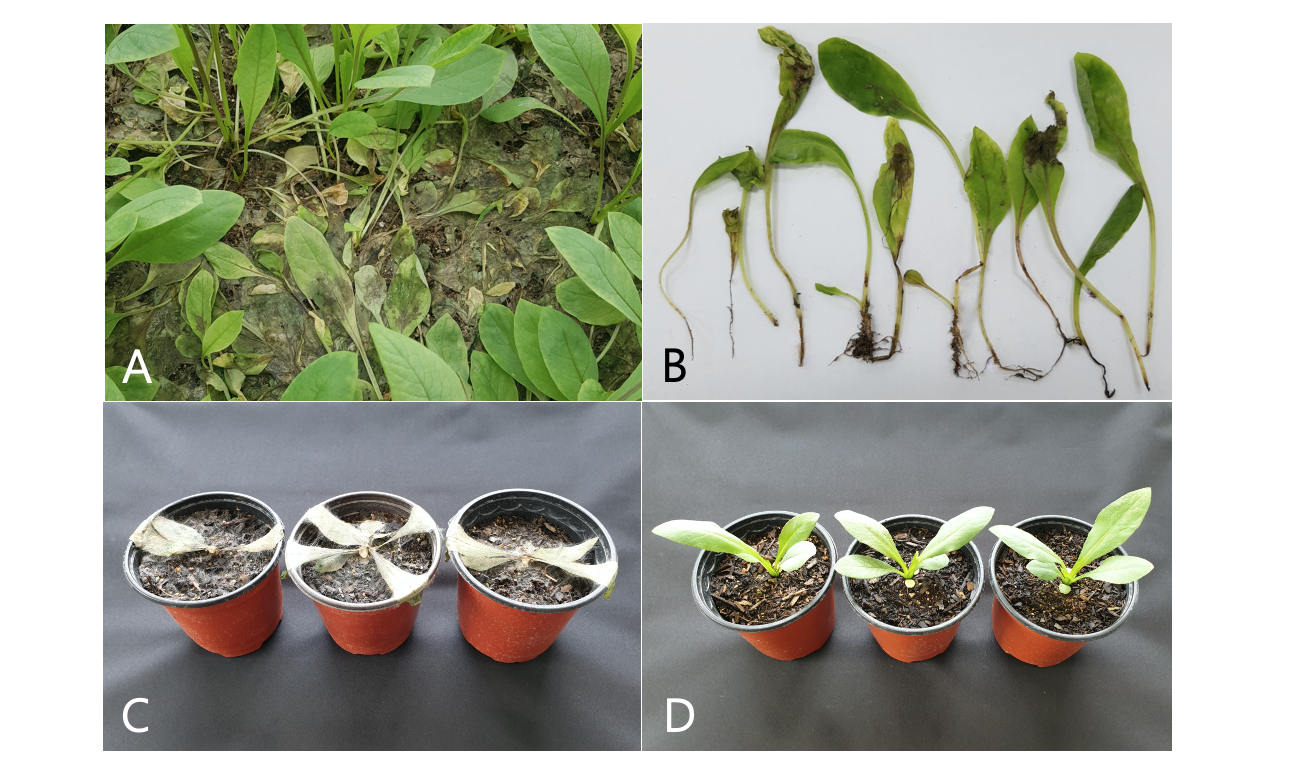
Indian lettuce (Lactuca indica L.) is a biennial herb and known to be mainly distributed in east and southwest Asia. The wild plant has been grown in Korea as a vegetable by farmers who grow the plant in vinyl greenhouses. Damping-off symptoms were frequently observed on young plants of Indian lettuce grown in a farmer's vinyl greenhouse located in Goseong, Gangwon province of Korea during a disease survey in June 2019. The symptoms initially manifested on the plant roots and stems at the soil surface. The infected plant tissues receded and were thin, discolored, and rotten. Diseased plants fell down and later blighted (Fig. 1A and 1B). Three sites were observed in the greenhouse, and 100 plants at each site were investigated for the disease incidence. The incidence of diseased plants was 30-50%.
Rhizoctonia sp. was frequently isolated from the diseased plants of Indian lettuce. Four isolates of Rhizoctonia sp. were obtained from lesions of the diseased plants and identified as Rhizoctonia solani Kühn based on the morphological characteristics as per descriptions from previous studies [1,2].
The isolates were evaluated to classify anastomosis groups using tester isolates of R. solani as previously described [3]. The R. solani tester isolates AG-1 (KACC 40101), AG-2-1 (KACC 40119), AG-2-2 (KACC 40125), AG-3 (KACC 40138), AG-4 (KACC 40139), and AG-5 (KACC 40146) were obtained from the Agricultural Microbiology Division, National Institute of Agricultural Sciences in Korea. All the tested isolates were classified as R. solani AG-4. Anastomosis reactions between the tested isolate and the tester isolate of R. solani AG-4 are shown in Fig. 2A. Mycelia of the isolates cultured on potato dextrose agar (PDA) were whitish light brown (Fig. 2B). Sclerotia were absent or rarely formed on the medium.
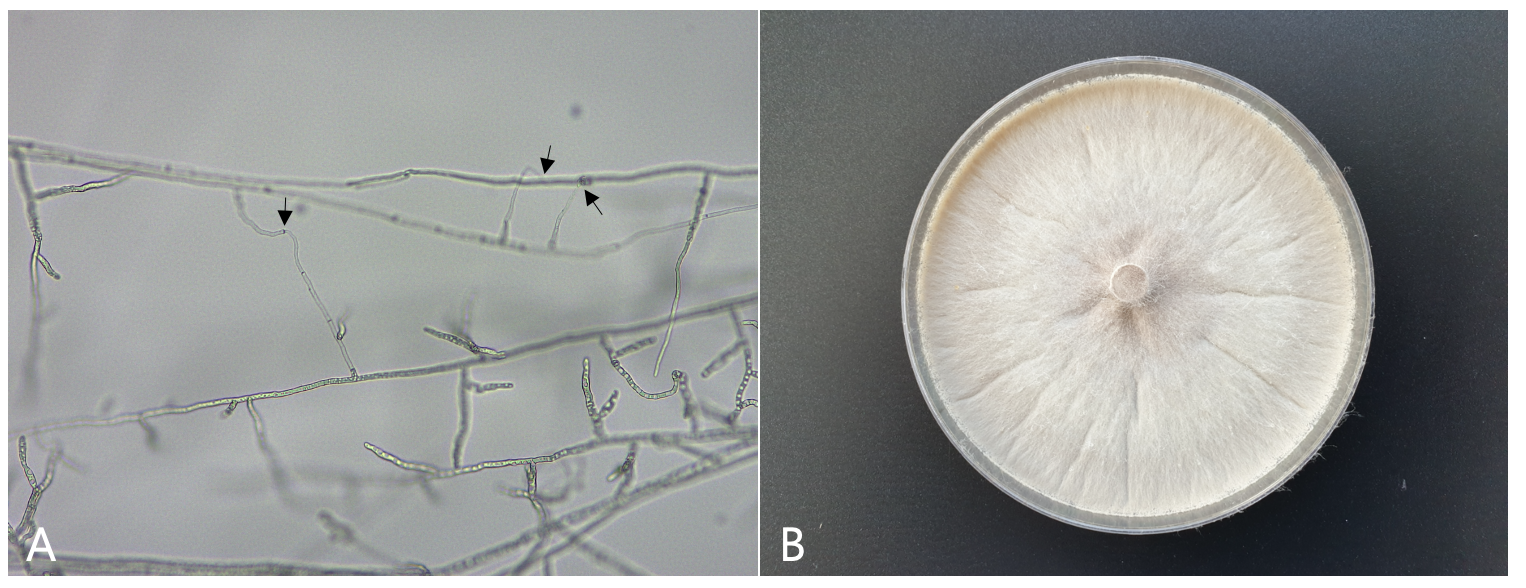
Fig. 2.Anastomosis test of Rhizoctonia solani isolate from Indian lettuce and cultural appearance of the isolate. A,anastomosis reactions between the tested isolate (left) and the tester isolate (right) of R. solani AG-4 observed by light microscope. Arrows indicate points of hyphal anastomosis; B, a colony of R. solani AG-4 isolate grown on PDA at 25℃ for 10 days.
Three isolates of R. solani AG-4 were tested for pathogenicity on Indian lettuce using artificial inoculation. Mycelial disks of 6 mm in diameter were cut from the margins of actively growing cultures of each isolate on PDA and placed at the soil surface level of 21-day-old Indian lettuce plants grown in circular plastic pots (height, 9 ㎝; upper diameter, 10 ㎝; lower diameter, 7 ㎝) in the vinyl greenhouse. Inoculated plant pots were placed in plastic boxes (60 ㎝ × 43 ㎝ × 33 ㎝) with 100% relative humidity at room temperature (24-26 ℃) for two days. Then, the inoculated plant pots were removed from the boxes and kept indoors. The virulence of the isolates was rated based on the degree of damping-off symptoms five days after inoculation. The inoculation test was performed in three replicates.
All the tested isolates of R. solani AG-4 induced damping-off symptoms on the inoculated plants (Fig. 1C), whereas no symptoms were observed on the control plants (Fig. 1D). The symptoms induced by the artificial inoculation of the plants were similar to those observed in plants from the vinyl greenhouse investigated. The isolates which induced symptoms on the plants were re-isolated from the lesions.
R. solani is reported to cause damping-off in many crops [4-6]. Indian lettuce is reported as a R. solani host in Thailand [7]. However, there has been no description of damping-off disease on Indian lettuce caused by the fungus. This is the first report of R. solani AG-4 causing damping-off in Indian lettuce.