Introduction
Penicillium is one of the most common fungal genera isolated from terrestrial environments, such as soil, indoors, plants, and food products. They play important ecological roles as decomposers, pathogens, and producers of a variety of molecules, such as bioactive compounds, enzymes, and mycotoxins [1,2]. Recently, Penicillium research has been expanded to various habitats, including marine (mudflats, sea sand, sea water, and algae) [3,4] and extreme environments (Antarctica, hot springs, saline soils, and deep seas) [5]. Many new species have been reported in ecological and biodiversity studies of specific substrates and habitats [6-8] and have increased significantly over the past decade [9].
Penicillium species are readily identified at the genus level according to their conidiophores and conidia, whereas they are difficult to identify at the species level because of their morphological plasticity under growth conditions and similar characteristics among closely related species [10]. Recently, the taxonomy of Penicillium has changed dramatically based on phylogenetic analysis. The description method of the species has been standardized, including morphology and multigene phylogenetic analysis using the nuclear rDNA internal transcribed spacer (ITS), β-tubulin (BenA), calmodulin (CaM), and RNA polymerase II second largest subunit (rpb2). In special, ITS and BenA have been recommended as molecular markers for Penicillium identification [10]. To date, Penicillium has been subdivided in two subgenera, 32 sections, 89 series, and 483 species worldwide [9]. Although the number of new and unrecorded species of Penicillium is continuously increasing [11-14], only about 24% of the described species have been found in South Korea [15]. Moreover, we have recently reported many potential new species and recorded species from mudflats and sea sand in the intertidal zones in South Korea using isolation and next generation sequencing (NGS) [4]. Therefore, it is necessary to expand our research toward new substrates and habitats using various methods, including NGS, in order to discover new species and unrecorded species.
As part of the projects organized by the Marine Fungal Resource Bank (MFRB) and the National Research Foundation of Korea (NRF), we discovered three unrecorded species from different series during the investigation of Penicillium diversity in marine and terrestrial environments. The main objective of this study was to describe these three unrecorded Penicillium species based on morphological characteristics and phylogenetic analysis of the BenA and CaM loci. They were identified as P. amaliae, P. infrabuccalum, and P. manginii.
Materials and methods
Sample collections and isolation
Using previously described methods [16], three strains were isolated from mudflats and one from seaweeds. Briefly, 5 g of mudflats was serially diluted (1/10 and 1/100) with sterilized seawater, and 100 μL of each dilution was plated on dichloran rose bengal chloramphenicol (DRBC, Difco-Becton Dickinson, MD, USA) agar. For fungal isolation from seaweed, samples were washed with seawater to remove surface debris, cut into discs of approximately 5-mm in diameter, and placed on DRBC agar. All plates were incubated at 25℃ for 7 days. Isolated fungal strains were transferred to potato dextrose agar (PDA; Difco-Becton Dickinson, MD, USA) with seawater. Each strain was stored in 20% glycerol at -80℃ at the Seoul National University Fungus Collection (SFC) (Table 1).
Table 1. Summary and GenBank accession numbers for Penicillium strains isolated from marine environments.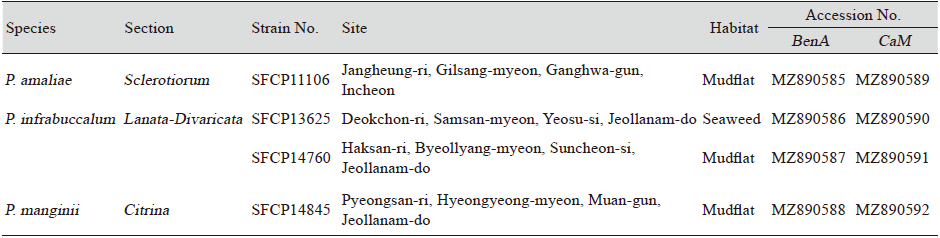
|
|
BenA: β-tubulin; CaM: Calmodulin |
DNA extraction, amplification, and sequencing
Genomic DNA was extracted using an AccuPrep Genomic DNA Extraction Kit (Bioneer, Daejeon, Korea) according to the manufacturer’s instructions. BenA and CaM were amplified using previously described methods [17]. The polymerase chain reaction (PCR) products were purified using an ExpinTM PCR Purification Kit (GeneAll Biotechnology, Seoul, Korea) according to the manufacturer’s instructions. DNA sequencing was performed bidirectionally at Macrogen (Seoul, South Korea) on an ABI Prism 3730XL analyzer (Applied Biosystems, Foster City, CA, USA) using the PCR primers.
Phylogenetic analysis
The sequences were manually assembled, proofread using MEGA5 [18], and deposited in GenBank (accession Nos. are listed in Table 1). Molecular identification was performed in two steps. First, the section of the strains were determined by comparing the BenA sequence of each isolated strain and those from several type strains. Next, each strain was identified to the species level by analyzing the combined dataset of the two loci (BenA and CaM) with type species from each section. P. roqueforti CBS 221.30, P. glabrum CBS 125543, and P. corylophilum CBS 312.48 were used as the outgroups for series Sclerotiorum, Simplicissima, and Westlingiorum, respectively [9]. Multiple alignments were performed with MAFFT v.7 [19] using the default settings. Maximum likelihood analyses were conducted using RAxML [20] implemented on the CIPRES web portal [21], using the general time reversible (GTR)+G model with 1000 bootstrap replicates.
Morphological analysis
The morphology of the three unrecorded species was observed using previously described methods on five different culture media: creatine sucrose agar (CREA), Czapek yeast autolysate agar (CYA; Difco, Sparks, MD, USA), dichloran 18% glycerol agar (DG18), malt extract agar (MEA; Oxoid, Hampshire, UK), and yeast extract sucrose agar (YES; Difco, Sparks, MD, USA) [22]. The Methuen handbook of color was used for determining the color names and alphanumeric codes for the description of each culture [23]. Microscopic features were observed under a Nikon 80i compound light microscope (Nikon, Tokyo, Japan) using colonies grown on MEA.
Results
Isolated strains were identified as P. amaliae in the series Sclerotiorum (1 strain), P. infrabuccalum in Simplicissima (2), and P. manginii in Westlingiorum (1) based on the combined dataset from BenA and CaM (Fig. 1).
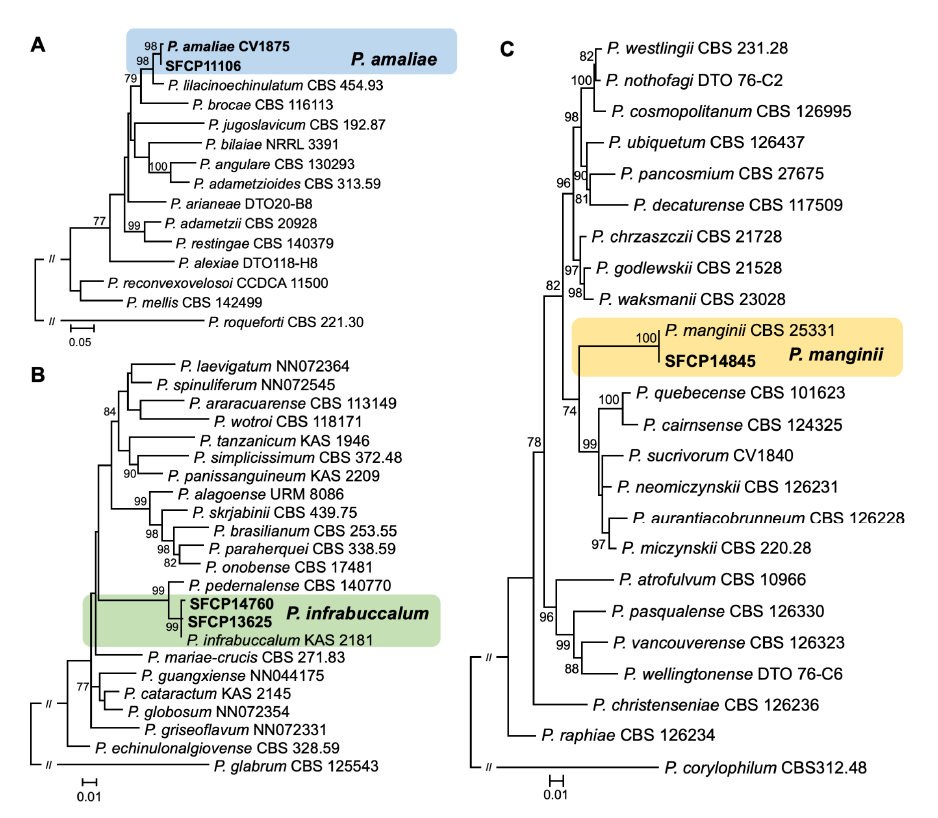
Fig. 1. Maximum likelihood (ML) phylogenetic trees of series (A) Sclerotiorum, (B) Simplicissima, and (C) Westlingiorum within Penicillium based on the combined data set of BenA and CaM. Bootstrap scores>70 are presented at the nodes. The scale bar represents the number of nucleotide substitutions per site. The unrecorded Penicillium species are accented in color boxes.
SFCP11106, classified in series Sclerotiorum sect. Sclerotiorum, formed a monophyletic group with P. amaliae CV1875 (type strain) (sequence similarity for BenA=99.1% and CaM=100%; bootstrap support=98%). Whereas SFCP13625 and SFCP14760, classified in series Simplicissima sect. Lanata-Divaricata, formed a monophyletic group with P. infrabuccalum KAS 2181 (type strain) (sequence similarity for BenA=99.8-100% and CaM=99.6-100%; bootstrap support=99%), and SFCP14845, classified in series Westlingiorum sect. Citrina, formed a monophyletic group with P. manginii CBS 253.31 (type strain) (sequence similarity for BenA=100% and CaM=100%; bootstrap support=100%).
Taxonomy
Penicillium amaliae Houbraken & K. Jacobs (2013) (Fig. 2)
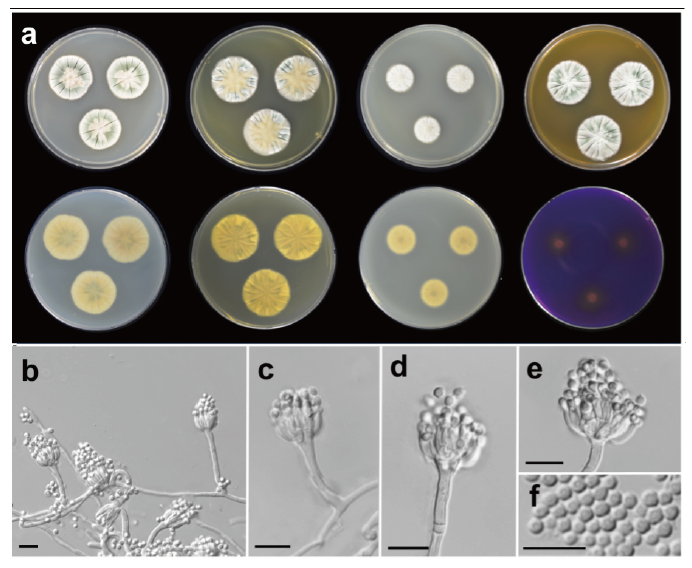
Fig. 2. Morphological characteristics of Penicillium amaliae SFCP11106. (a) Colonies after 7 days at 25℃, from left to right (top row) Czapek yeast autolysate agar (CYA), yeast extract sucrose agar (YES), dichloran 18% glycerol agar (DG18), malt extract agar (MEA); (bottom row) CYA reverse, YES reverse, DG18 reverse, creatine sucrose agar (CREA) reverse; (b-e) Conidiophores; (f) Conidia (scale bar: b-f=10 μm).
Description: Colony diam, 7 d, in mm: CYA 25℃ 27-30; CYA 15℃ 9-10; CYA 30℃ 23-25; CYA 37℃ no growth; MEA 25℃ 25-29; YES 25℃ 28-30; DG18 25℃ 19-20; CREA 25℃ 14-16 (Fig. 2).

Fig. 2. Morphological characteristics of Penicillium amaliae SFCP11106. (a) Colonies after 7 days at 25℃, from left to right (top row) Czapek yeast autolysate agar (CYA), yeast extract sucrose agar (YES), dichloran 18% glycerol agar (DG18), malt extract agar (MEA); (bottom row) CYA reverse, YES reverse, DG18 reverse, creatine sucrose agar (CREA) reverse; (b-e) Conidiophores; (f) Conidia (scale bar: b-f=10 μm).
Colony characteristics: CYA, 25℃, 7 d: Colonies moderately deep, radially sulcate; margins low, narrow, entire; mycelia white; texture velvety; sporulation moderately dense; conidia dull green (27E3) to grayish green (27B3); exudates clear to almost yellowish brown; soluble pigments absent; reverse color light orange (5A5) to pale yellow (2A3). MEA, 25℃, 7 d: Colonies low, radially sulcate; margins low, narrow, entire; mycelia white; texture velvety; sporulation moderate to dense; conidia grayish green (26B3); exudates absent; soluble pigments absent; reverse color deep orange (6A8). YES, 25℃, 7 d: Colonies moderately deep, radially sulcate; margins low, narrow, entire; mycelia white; texture velvety; sporulation sparse to moderate; conidia grayish green (26B4); exudates absent; soluble pigments absent; reverse color light grayish yellow (3B5). DG18, 25℃, 7 d: Colonies low, radially sulcate; margins low, wide, entire; mycelia white; texture floccose; sporulation absent to sparse; conidia grayish turquoise (24B4); exudates absent; soluble pigments absent; reverse color grayish yellow (3B3). CREA, 25℃, 7 d: Growth poor; acid production weak.
Conidiophores monoverticilate; stipes smooth walled, 16-59×2-3 μm, phialides ampulliform, 9-19 per stipe, 6.3-16.5×1.8-2.9 μm. Conidia rough walled, globose to subglobose, 2.3-3.0×1.9-2.8 μm. Sclerotia absent; asci and ascospores not observed.
Strains examined: SFCP11106
Note: P. amaliae is phylogenetically similar to P. lilacinoechinulatum. Both species can be distinguished by their different growth rates on CYA at 30℃ and acid production on CREA: P. amaliae grows slower than P. lilacinoechinulatum on CYA at 30℃ after 7 d (23-25 vs. 30-33 mm) [24]. P. amaliae produced a weak acid on CREA, whereas P. lilacinoechinulatum produced strong acid on CREA. Interestingly, the strain in this study showed no growth on CYA at 37℃, whereas the type strain had a low growth rate of 3-11 mm.
Penicillium infrabuccalum Visagie, David Clark & Seifert (2016) (Fig. 3)
Description: Colony diam, 7 d, in mm: CYA 25℃ 53-63; CYA 15℃ 18-21; CYA 30℃ 52-56; CYA 37℃ no growth; MEA 25℃ 33-39; YES 25℃ 48-51; DG18 25℃ 21-23; CREA 25℃ 38-40 (Fig. 3).
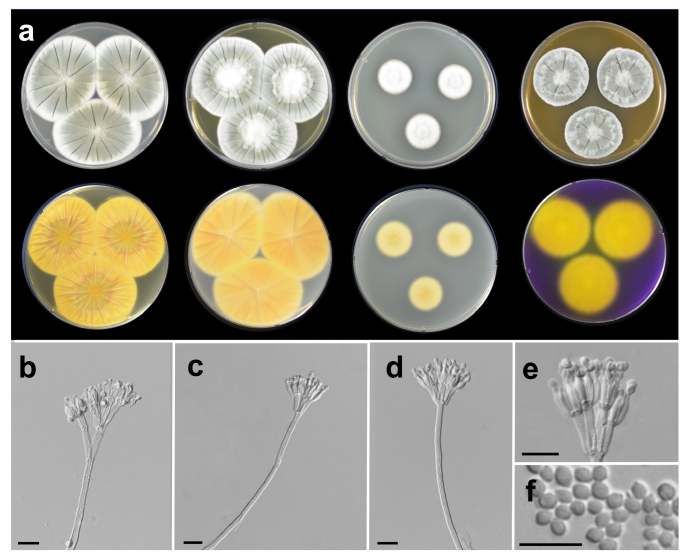
Fig. 3. Morphological characteristics of Penicillium infrabuccalum SFCP13625. (a) Colonies after 7 days at 25℃, from left to right (top row) Czapek yeast autolysate agar (CYA), yeast extract sucrose agar (YES), dichloran 18% glycerol agar (DG18), malt extract agar (MEA); (bottom row) CYA reverse, YES reverse, DG18 reverse, creatine sucrose agar (CREA) reverse; (b-e) Conidiophores; (f) Conidia (scale bar: b-f=10 μm).
Colony characteristics: CYA, 25℃, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white; texture floccose; sporulation moderately dense; conidia grayish green (25B3); exudates; soluble pigments absent; reverse color light orange (5A5), light yellow (4A4) at margin. MEA, 25℃, 7 d: Colonies low to moderately deep, radially sulcate; margins low, narrow, entire; mycelia white; texture floccose; sporulation moderately dense; conidia dull green (25D4) at center, grayish green (25B3) elsewhere; exudates absent; soluble pigments absent; reverse color orange (5B8). YES, 25℃, 7 d: Colonies moderately deep, radially sulcate; margins low, wide, entire; mycelia white; texture floccose at center, velvety elsewhere; sporulation sparse to moderately dense; conidia grayish green (27E4) to greenish gray (26B2); exudates absent; soluble pigments absent; reverse color brownish orange (5C6) to grayish yellow (4C3) at center, deep yellow (4A8) elsewhere. DG18, 25℃, 7 d: Colonies low, radially sulcate; margins low, narrow, entire; mycelia white; texture floccose; sporulation sparse; conidia greenish white (25A2); exudates absent; soluble pigments absent; reverse color light yellow (4A5) at center, pale yellow (4A3) elsewhere. CREA, 25℃, 7 d: Growth moderate; acid production absent.
Conidiophores biverticillate, terverticillate; stipes rough walled, 100-410×2-3 μm; branches 24-37 μm; metulae divergent, 2-5 per stipe, 9.7-13×2.5-3.5 μm; phialides ampulliform, 4-10 per metula, 6.1-11.2×1.6-3.2 μm. Conidia finely rough walled, subglobose to ellipsoidal, 2.5-3×2-3 μm. Sclerotia absent; asci and ascospores not observed.
Strains examined: SFCP13625 and SFCP14760.
Note: P. infrabuccalum is phylogenetically similar to P. pedernalense. P. infrabuccalum is characterized by no acid production on CREA, whereas P. pedernalense produces strong acid on CREA [25]. Korean P. infrabuccalum tends to grow faster than the type strain KAS 2181 of P. infrabuccalum on CYA and MEA at 25℃.
Penicillium manginii Duché & Heim (2011) (Fig. 4)
Description: Colony diam, 7 d, in mm: CYA 25℃ 29-31; CYA 15℃ 14-16; CYA 30℃ no growth; CYA 37℃ no growth; MEA 25℃ 28-30; YES 25℃ 35-38; DG18 25℃ 18-19; CREA 25℃ 12-15 (Fig. 4).
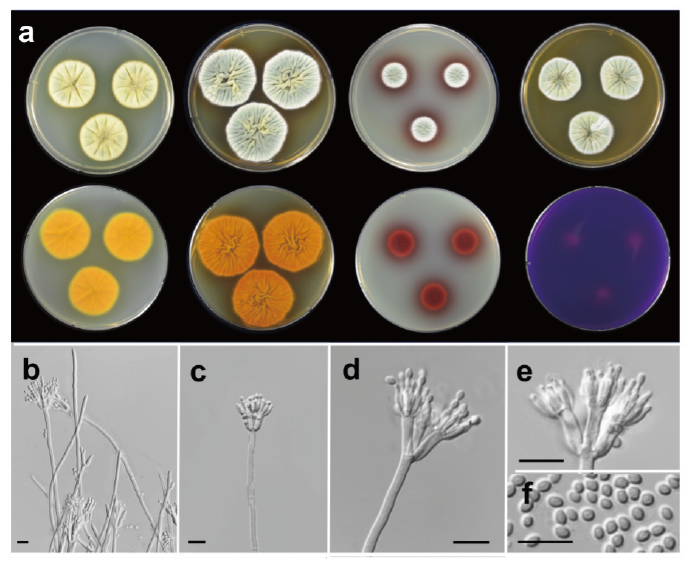
Fig. 4. Morphological characteristics of Penicillium manginii SFCP14845. (a) Colonies after 7 days at 25℃, from left to right (top row) Czapek yeast autolysate agar (CYA), yeast extract sucrose agar (YES), dichloran 18% glycerol agar (DG18), malt extract agar (MEA); (bottom row) CYA reverse, YES reverse, DG18 reverse, creatine sucrose agar (CREA) reverse; (b-e) Conidiophores; (f) Conidia (scale bar: b-f=10 μm).
Colony characteristics: CYA, 25℃, 7 d: Colonies moderately deep, radially sulcate; margins low, narrow, entire; mycelia light yellow (2A4); texture velvety; sporulation moderately dense; conidia grayish green (27B4); exudates absent or yellow; soluble pigments yellow; reverse color deep orange (5A8). MEA, 25℃, 7 d: Colonies low, radially sulcate; margins low, narrow, entire; mycelia white; texture velvety; sporulation poor to moderately dense; conidia grayish green (3B4); exudates clear; soluble pigments absent; reverse color deep orange (5A8). YES, 25℃, 7 d: Colonies deep, randomly furrowed; margins low, narrow, entire; mycelia white; texture velvety; sporulation moderately dense; conidia grayish green (26B4); exudates absent; soluble pigments reddish brown (9D8); reverse color reddish brown (8D8). DG18, 25℃, 7 d: Colonies low; margins low, narrow, entire; mycelia white; texture velvety; sporulation moderately dense; conidia grayish green (26B3); exudates absent; soluble pigments brownish red (10D8); reverse color violet brown (10F8). CREA, 25℃, 7 d: Growth poor; acid production weak; reverse color red (11A6).
Conidiophores biverticillate; stipes smooth to finely rough walled, 150-450×2-4 μm; metulae 2-5 per branch, 8.0-11.9×2.7-3.8 μm; phialides ampulliform, 6-8 per metula, 5.0-8.3×1.4-2.8 μm. Conidia smooth walled, subglobose to ellipsoidal, 2.3-3.6×1.2-2.4 μm. Asci and ascospores not observed.
Strains examined: SFCP14845.
Note: P. manginii is morphologically similar to P. vancouverense. Both species can be distinguished by their growth rate and soluble pigment [26]. P. manginii grows faster than P. vancouverense on MEA and YES at 25℃. P. manginii produces a reddish brown color on YES, whereas P. vancouverense does not produce any soluble pigment. Korean P. manginii tends to grow slower than the type strain CBS 253.31 on CYA at 25℃.
Discussion
Penicillium species are one of the most common fungi genera found in various substrates of marine environments, such as sea sand, seaweeds, and sailfin sandfish egg masses [3,27,28]. We identified three Penicillium species new to South Korea, that were isolated from marine environments; according to phylogenetic analyses based on the BenA and CaM loci, these species are P. amaliae, P. infrabuccalum, and P. manginii. Each of these Penicillium species was included in different sections and series: P. amaliae belonged to series Sclerotiorum, sect. Sclerotiorum; P. infrabuccalum to series Simplicissima, sect. Lanata-Divaricata; and P. manginii to series Westlingiorum, sect. Citrina.
The series Sclerotiorum included in sect. Sclerotiorum consists of 17 species and is characterized by monoverticillate conidiophores, moderately fast colony growth, dull green to gray-green conidia, and globose to ellipsoidal conidia [9]. In South Korea, 11 Penicillium species have been reported, including P. acidum, P. austrosinicum, P. cainii, P. daejeonium, P. exsudans, P. jacksonii, P. johnkrugii, P. mallochii, P. maximae, P. sclerotiorum, and P. ulleungdoense [14]. They have been isolated from various substrates in marine and terrestrial environments [29-33]. P. amaliae was previously reported in the infructescence of Protea repens and insect larva [24]. Although SFCP11106 from mudflats showed a difference in mycelial growth at 37℃ on CYA compared with that of the type strain of P. amaliae, there were no differences in other morphological characteristics [24]. SFCP11106 formed a monophyletic group with the type strain of P. amaliae and was clearly different from other species in the series Sclerotiorum, based on BenA and CaM sequence analysis. Therefore, the strain could be confidently identified as P. amaliae. The series Simplicissima included in sect. Lanata-Divaricata consists of 20 species. Although the species in the series Simplicissima are morphologically characterized by biverticillate or divaricate conidiophores, colony growth at 37℃, gray-green or dull green conidia, and conidia of various sizes and shapes, classification of the series is difficult to establish based on morphological features [9]. In South Korea, six species have been reported, including P. brasilianum, P. echinulonalgiovense, P. globosum, P. onobense, P. paraherquei, and P. simplicissimum [15,34]. They were isolated from terrestrial environments, such as fresh water, indoors, and soil [4,29,34-36]. SFCP13625 and SFCP14760 were isolated from mudflats and seaweeds in marine environments and showed slight differences in sporulation and growth rate with previously reported P. infrabuccalum [25]. However, based on BenA and CaM sequence analysis and other morphological features, the strains were identified as P. infrabuccalum.
The series Westlingiorum included in sect. Citrina comprises 22 species and is morphologically characterized by biverticillate conidiophores, no growth at 37℃, and blue green to grayish green conidia [9]. A total of seven species have been reported in South Korea, including P. cairnsense, P. decaturense, P. miczynskii, P. pasqualense, P. raphiae, P. waksmanii, and P. westlingii [12,15]. With the exception of P. westlingii, most species have been isolated from soils in marine and terrestrial environments [4,12,16,37,38]. SFCP14845 was isolated from mudflats, and although it grew slower than the type strain of P. manginii on CYA at 25℃, most of its morphological features were similar to those of the type strain [26]. SFCP14845 formed a well-supported monophyletic group with P. manginii CBS 253.31 (type strain). Therefore, we identified it as P. manginii.
In conclusion, we confirmed the presence of three unrecorded Penicillium species in South Korea using morphological and molecular approaches. The results of this study contribute to the update of the fungal inventory in this country. Continuous fungal surveys, along with accurate identification based on morphological and molecular characteristics, will increase the possibility of discovering more unrecorded species or even new ones.


