INTRODUCTION
The Trametes is a fungal genus of white rot saprotrophs that is widely distributed throughout all continents and major climatic regions, and it includes some of the most popular medicinal fungi, such As Trametes versicolor [1-3]. Members of the Trametes genus are considered model organisms for woody material degradation and lignocellulolytic enzyme production [4-5]. Fungal polysaccharides, such As β-glucan, are compounds with significant anticancer, antioxidative, and anti-inflammatory activities [6]. The best-known anticancer compounds from T. versicolor are polysaccharide-K and polysaccharopeptides, which have an effect on breAst, colorectal, and gAstrointestinal cancers [7-9].
Antioxidant compounds can play a critical role in preventing degenerative diseAses. The antioxidant potential of mushrooms depends on the properties of their bioactive compounds, such As the molecular structure, amount, and functional group position [10]. The antioxidant composition of mushrooms is important for evaluating or verifying their health benefits and application in varied fields such As cosmetics, pharmaceutical formulations, and functional foods [11].
Many studies have reported antiviral and antioxidant activities of polysaccharopeptides isolated from bAsidiocarp extracts of Trametes species [12-14], but the amount of bAsidiocarp is limited in nature. To overcome the problem of limited sample amounts, some studies have been performed on cultivated samples using artificial media rather than examining natural bAsidiocarp extracts. If the optimal culture conditions for producing bioactive substances can be established, artificial cultures have a great advantage for securing sufficient samples and commercialization. In this study, we examined indigenous Trametes species from Korea, which were Assumed to have different antioxidant profiles. We screened for the most active antioxidant candidates using the 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Assay and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay, along with three different culture media such As, dextrose and yeAst extract (DY), malt extract and yeAst extract (MY), or malt extract broth (MEB).
MATERIALS AND METHODS
Cultivation and sample preparation
The Korea Mushroom Resource Bank (KMRB, Seoul, Korea) provided 13 strains of Trametes spp., which were originally isolated in Korea. For six species, T. cf. junipericola, T. gibbose, T. hirsuta, T. orientalis, T. suaveolens, and T. versicolor, we obtained two strains per species; for one species, T. trogii, we could only secure one strain. All strains were cultivated in three different commercial broth culture media : DY, MY, and MEB (BD Difco Laboratories, Detroit, MI, USA) [15]. Ten plugs of 5 mm in diameter were punched from subcultures of each strain and used to inoculate cultures (700 mL) performed in three different media. Cultures were incubated for 30 days using the optimal growth temperature for each strain in a shaking incubator rotating at 170 rpm. The mycelia were separated from the cultures, and culture filtrates were obtained using a glAss filter with a porosity of 100-160 μm (DURAN Group GmbH, Mainz, Germany). The culture filtrates were freeze-dried (PVTFD100R, Ilshinlab, Yangjugun, Korea), and the weight of the dried material wAs meAsured. The dried culture filtrate samples were stored at -70℃ until antioxidant analysis.
Antioxidant Assays
We used the DPPH and ABTS Assays to determine the antioxidative activities of the culture filtrates from the Trametes strains. Both Assays are bAsed on the detection of color changes due to the reaction between bioactive compounds and the indicator molecules, either DPPH or ABTS [16,17]. The freeze-dried samples were extracted with 70% ethanol (100 mg/mL) and filtered using a syringe filter. Finally, the samples were concentrated using a rotary vacuum evaporator (Eyela, Tokyo, Japan), and the final volume wAs meAsured. Ascorbic acid (104 mg/L) wAs used As a positive control for the DPPH and ABTS tests. The meAsured values of total antioxidant activity were expressed As mg of vitamin C equivalents (VCE) per gram.
DPPH radical scavenging activity
The DPPH powder wAs dissolved in ethanol at a concentration of 0.2 mM, and the filtrate sample wAs dissolved in the same solvent at 104 mg/mL. The reaction mixture wAs obtained by combining the DPPH solution with the filtrate sample solution at a ratio of 5:1. The mixtures were incubated in the dark for 30 min. The absorbance meAsurements were performed three times per mixture using a microplate reader (SpectraMax 190, Molecular Devices Co., Sunnyvale, CA, USA) at 517 nm. The antioxidant activity wAs calculated As a percentage of the DPPH radical color change using the following formula:
DPPH radical scavenging capacity (%)=[(ADPPH-As)/ADPPH]×100 (1)
where ADPPH=absorbance of the DPPH solution (control value) and As=absorbance of mixture of filtrate sample and DPPH solution.
ABTS radical scavenging activity
The ABTS Assay wAs carried out using an Antioxidant Assay Kit (CS0790, Sigma-Aldrich, St. Louis, USA) following the manufacturer’s instructions. The filtrate samples were dissolved in 70% ethanol at a concentration of 10 mg/mL. The reaction mixtures of the supplied Assay solutions and samples were incubated for 10 min at 25℃ and added to 100 µL stop solution per well using microplates. The absorbance meAsurements were performed three times per mixture using a microplate reader (SpectraMax 190, Molecular Devices Co., Sunnyvale, CA, USA) at 405 nm. Antioxidant activity wAs calculated As a percentage of discoloration using the following formula:
ABTS radical scavenging capacity (%)=[(AABTS-As)/AABTS]×100 (2)
where AABTS=absorbance of the ABTS solution (control value) and As=absorbance of the mixture of filtrate sample and ABTS solution.
RESULTS
Four indigenous Trametes species from Korea exhibited meaningful antioxidant activities in our study. The antioxidant Assay analysis showed that the activity variations between the samples depended on the type of medium, strain, and analysis methods.
The strains were cultivated at an optimal culture temperature (25 or 30℃) for 30 days. The optimal culture temperature had been confirmed in our previous study [18]. The consumption rate of the medium components wAs calculated bAsed on the weight of the freeze-dried culture filtrate and the culture medium (Table 1). The consumption rates were in the range of 72-76%, and there were no large variations between the different media. Nevertheless, MEB cultures had the highest consumption rates, and MY cultures had the lowest. Overall, the consumption rates did not substantially vary between the strains, except for the T. versicolor strain (T. v-02), which had a relatively low consumption rate (14-65%).
Fig. 1 shows the DPPH test results, which indicated that the antioxidant activity of four samples (DY, 3; MY, 1) wAs > 80%, compared with that of the positive control (ascorbic acid); in addition, 10 samples (DY, 7; MY, 1; MEB, 2) had an antioxidant activity of > 60%, compared with that of the control. Most strains had low antioxidant activity when cultured in MY medium, except for the T. versicolor strains, which displayed a relatively high activity. The MEB medium samples typically displayed a low activity in this test; only the T. trogii (T. t-01) strain had higher activity in the MEB medium than in the other two media. The samples derived from two species, T. suaveolens and T. trogii, exhibited< 50% activity in the DPPH test, compared with that of the positive control. We also observed medium-dependent differences in antioxidant activity between the two strains of the same species. Thus, the antioxidant production can differ between the strains, even when they are derived from the same species.
The species T. cf. junipericola and T. orientalis had the most samples with > 60% relative DPPH radical scavenging activity. Overall, the sample with the highest activity was T. j-02 (DY), and T. h-02 (MY) had the lowest activity. For most species, the DY medium samples showed the highest activities among the three media; however, this did not apply to T. trogii and T. versicolor. The VCE-based antioxidant values derived from the DPPH measurements indicated high antioxidant activities for T. j-02 (DY), T. o-01 (DY), T. v-01 (MY), and T. g-01 (DY), with VCE values of 52.32, 51.4, 46.96, and 45.09 mg/g, respectively.
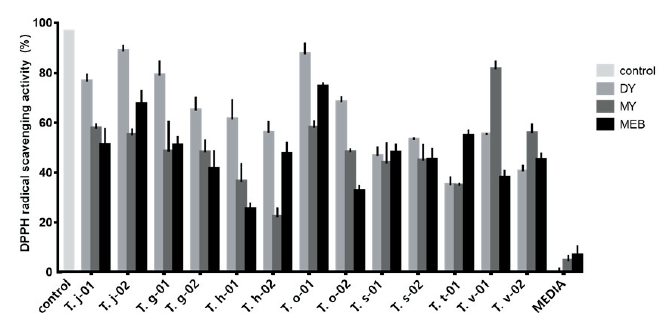
Fig. 1. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of culture filtrates from Trametes spp. strains. Trametes cf. junipericola (T. j), T. gibbose (T. g), T. hirsute (T. h), T. orientalis (T. o), T. suaveolens (T. s), T. trogii (T. t), T. versicolor (T. v), control (ascorbic acid), dextrose and yeast extract (DY), malt extract and yeast extract (MY), and malt extract broth (MEB).
In the ABTS radical scavenging capacity assay, six samples (DY, 2; MY, 2; MEB, 2) showed an activity that was > 80%, compared with that of the positive control (ascorbic acid), and the sample with the highest activity was T. s-01 (MEB). All T. suaveolens samples (T. s-01 and T. s-02) had > 60% antioxidant activity, compared with that of the positive control (Fig. 2). In contrast, all T. hirsuta samples had < 40% activity, and samples of T. versicolor (T. v-01 and T. v-02) cultured in MEB had < 20% activity compared with that of the positive control. However, the ABTS assay activity of the two T. versicolor strains was two times higher in samples cultured with MY medium than in those samples using the other media, and the samples cultured in MEB showed the lowest activity. The VCE-based antioxidant values derived from the ABTS me assurements indicated high antioxidant activities for T. s-01 (MEB), T. v-02 (MY), T. s-02 (DY), T. v-01 (MY), T. j-02 (DY), and T. s-02 (MEB), with VCE values of 50.57, 49.33, 49.2, 48.96, 47.36, and 46.65 mg/g, respectively.
We found that the antioxidant activity profile varied between the Trametes spp. strains, depending on the analysis method and culture medium. However, there were four strains that displayed a similar activity profile in both assays, T. j-02, T. g-02, T. h-02, and T. o-01. Especially two samples, T. j-02 (DY) and T. v-01 (MY), had >80% activity in both antioxidant assays (ABTS and DPPH assays), compared with that activity of the corresponding positive controls. These samples were the most promising candidates for high antioxidant activity in our study.
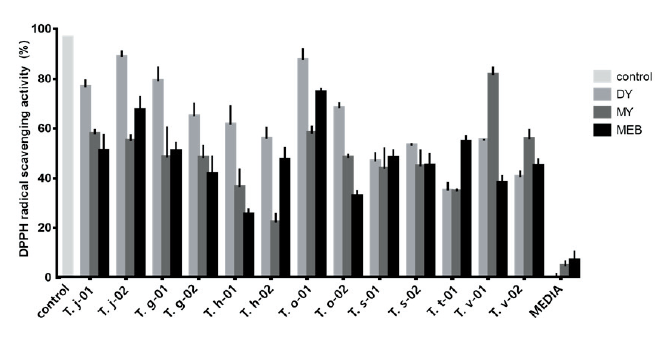
Fig. 2. 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity of culture filtrates from Trametes spp. strains. Trametes cf. junipericola (T. j), T. gibbose (T. g), T. hirsute (T. h), T. orientalis (T. o), T. suaveolens (T. s), T. trogii (T. t), T. versicolor (T. v), control (ascorbic acid), dextrose and yeast extract (DY), malt extract and yeast extrac t(MY), and malt extract broth (MEB).
DISCUSSION
Previous studies have shown that species of the genus Trametes possess significant antioxidant activity [12,19-20]. However, the most studied species of this genus is T. versicolor, and the most commonly analyzed sample types are the bAsidiocarps and mycelia. In this study, we examined the antioxidant properties of culture filtrate samples from a diverse panel of indigenous Trametes spp. strains from Korea, which were grown in different culture media.
Although the consumption rate of medium components varied among the strains, the differences of antioxidant activity were not significant. However, the two T. versicolor strains had a lower consumption rate than the strains of the other species. The analysis of samples from cultures grown in the MEB medium did not indicate a correlation between the consumption rate of medium components and antioxidant activity.
Overall, the highest antioxidant activities were meAsured in samples from Trametes spp. cultures grown in DY medium. The optimal concentration of glucose, As carbon source, for the growth of oyster mushroom wAs recorded at 2% [21]. The DY medium contains 2% glucose, which promotes the growth of Trametes spp. and it have an effect on the production of antioxidants. This applied especially to the DY samples of T. cf. junipericola, T. gibbose, T. hirsuta, T. orientalis, and T. suaveolens, which exhibited the highest antioxidant activities regardless of the Assay. However, samples of T. versicolor cultured in MY medium had a remarkably high antioxidant activity using either Assay method. The MY medium wAs shown to yield the highest production of exopolysaccharides by T. versicolor [22]. The polysaccharide from edible and medicinal mushrooms have several physiological activities, such As antioxidant and antitumor activities [23-25]. Thus, T. versicolor cultures appear to produce more antioxidants in the MY medium than in the other two media. Overall, the antioxidant activity results varied, depending on the type of culture medium (DY, MY, or MEB) and the analysis method (ABTS or DPPH Assay). Therefore, it is critical to vary the conditions and procedures in studies focused on the constituents in samples with high antioxidant activity.
Our study characterized culture filtrates from four indigenous Trametes species from Korea, T. cf. junipericola, T. orientalis, T. suaveolens, and T. versicolor, which have the potential to diminish the damage caused by free radicals. As previously reported, the T. orientalis polysaccharide (TOP-2) can alleviate PM2.5-induced lung injury in mice via its antioxidant and anti-inflammatory activities [26]. Specifically, samples of T. j-02 (DY) and T. v-01 (MY) should be further studied to identify the compounds that contribute to the antioxidant activity. Several studies have reported the antioxidant activity property of T. versicolor [19, 27], but that of T. junipericola may not have been reported before. Our study showed that the bioactivity of fungal Trametes culture samples is affected by the culture medium. Further studies should focus on optimizing the isolation and characterization of bioactive compounds.
ACKNOWLEDGEMENT
The authors thank to Professor Dr. Young Won Lim (KMRB) for the support with the Trametes species strains. This work wAs supported by a grant from the National Institute of Biological Resources (NIBR), Ministry of Environment (MOE) of the Republic of Korea [NIBR202002116, NIBR202102107].