INTRODUCTION
Chili pepper (Capsicum spp.) is an essential food ingredient in tropical and subtropical countries and the fourth major crop produced globally. Approximately 400 varieties of chili pepper are cultivated worldwide [1], and chili pepper is among the world’s most important spices as food additives. Chili peppers are frequently infected with phytopathogenic molds which produce dangerous mycotoxins due to cultural practices, transport, and post-harvest storage problems [2]. Colletotrichum acutatum and C. gloeosporioides are important anthracnose pathogens of a wide range of host plants worldwide. Chili anthracnose is a major constraint in chili pepper production, which frequently entails considerable economic losses [3]. Sclerotinia sclerotiorum is a necrotrophic parasitic fungus causing Sclerotinia stem rot (SSR) which is currently among the agronomic crop diseases that are most difficult to control [4]. Phytophthora cactorum has a considerably wide host range and Phytophthora blight of chili pepper is the most economically crucial disease in all cultivation regions [5], and this oomycete is particularly problematic in low-lying areas and under wet field conditions. Chili pepper is highly susceptible to many fungal diseases including damping-off, root rots and wilts. These diseases can attack chili pepper at any growth stage and are caused by several soil borne pathogens along with F. solani, Fusarium oxysporum [6].
Generally, chemical pesticides have been used to control fungal diseases, however, their indiscriminate use has resulted in adverse consequences for the environment and human health. Moreover, overuse of chemical pesticides may induce fungal tolerance to pathogenic microorganisms and insects. The effects of the alternating application of agricultural organic compounds and synthesized fungicides for managing chili pepper anthracnose have been examined in vitro and in the field. In previous studies, microbial agents (e.g., Bacillus subtilis) and agricultural organic materials (e.g., sulfur, Bordeaux mixture, and marine algae extracts) showed strong inhibition effects on C. acutatum [7].
Biological control, based on the use of microorganisms, may thus be an efficient and sustainable alternative for Capsicum cultivation and offers a series of additional benefits [8]. Bacillus is the most abundant genus in the rhizosphere, and effects of plant growth-promoting rhizobacteria (PGPR), including some Bacillus strains, have been known for many years [9]. Several strains of Bacillus sp. exert marked antibacterial and antifungal effects on phytopathogens. Bacillus species directly antagonize fungal pathogens through competition for nutrients and niches and by producing antifungal compounds (lipopeptides, antibiotics, and enzymes), and indirectly by inducing systemic resistance or by promoting plant growth through different mechanisms such as siderophore production [10]. Bacillus species also synthesize many potent amphiphilic and surfactant lipopeptides such as bacillomycins, iturins, and mycosubtilin; under modified culturing conditions, these bacteria also produce fengycins and plipastatin [11].
The present study investigated Bacillus strains isolated from field soil samples collected in Sunchang, Republic of Korea. To identify bacteria exerting antifungal effects, microbial extracellular enzyme activity, plant growth-promoting (PGP) activity, profiles of a carbohydrate fermentation test (API 50 CH) and API ZYM activity, time course profiling of cell growth and plant growth promoting effects were investigated.
MATERIALS AND METHODS
Isolation of bacteria from soil samples
Fifty bacterial strains were isolated from soil samples collected in Sunchang, Korea. Soil samples were placed in conical tubes and were kept at ambient temperature until culturing. Bacillus species were isolated using the serial dilution plating method. Briefly, 1 g of soil was mixed with 9 mL of sterilized 0.85% saline solution and aliquots of 100 µL of each dilution were transferred to Luria-Bertani (LB) agar (Difco, Detroit, MI, USA) and were incubated at 30℃ for 24 hr. Pure isolates were suspended in 10% skim milk stock solution and were stored at -80℃.
Determination of microbial extracellular enzymes
The amounts of extracellular enzymes produced by the isolates were determined using the agar well diffusion method. The isolates were grown on media containing 1% carboxymethyl-cellulose (JUNSEI Chemical Co., Ltd., Tokyo, Japan), 2% skim milk (Difco), and 1% soluble starch (JUNSEI Chemical Co., Ltd.) as substrates for the production of protease, cellulase, and amylase, respectively. Each isolate was inoculated in LB medium, was incubated at 30℃ for 48 hr, and was then centrifuged at 13,000 rpm for 30 min to collect the supernatant. After filtering the supernatant using a 0.45 μm syringe filter (Sartorius, Frankfurt, Germany), 100 µL of the supernatant was placed in each well (6 mm diameter) of plates which were incubated aerobically at 30℃ for 18 hr. After incubation, the diameter of the halo or the clear zone formed around the well was measured to assess enzyme activity. The five strains with the highest enzyme activities were selected for further examination.
Antifungal effects
Preliminary screening of the five selected strains was conducted with respect to the antifungal effects on phytopathogens including Colletotrichum acutatum KACC 40042, C. gloeosporioides KACC 40003, Sclerotinia sclerotiorum KACC 41065, Phytophthora cactorum KACC 40166, Fusarium oxysporum KCCM 11554, and F. solani which isolated from hydroponically cultured Panax ginseng using an agar well diffusion assay. In a dual culture assay using potato dextrose agar (PDA) medium (Difco), wells of 6 mm diameter were cut into each agar plate under hygienic conditions, and 100 µL of the supernatant was placed in each well. The plates were then incubated aerobically at 25℃ for seven days, and sizes of the clear zones were measured.
PGP characteristics
To evaluate the potential effect of plant growth-promoting rhizobacteria (PGPR) of isolates, PGP factors such as siderophore and indole-3-acetic acid (IAA) were analyzed. The siderophore production of the five strains was examined by a chrome azurole S (CAS) blue agar plate assay. CAS agar plates were prepared by mixing 100 mL of the CAS reagent with 900 mL of sterilized LB agar medium [12]. CAS mixture was prepared using 50 mL of solution A (60 mg chromazurol in 50 mL water), 10 mL of solution B (2.7 mg FeCl3·6H2O in 10 mM HCl), and 40 mL of solution C (73 mg hexadecyltrimethylammonium bromide in 40 mL water). The resulting dark blue mixture was autoclaved at 121℃ for 15 min. Subsequently, 20 μL of the culture medium of each strain was inoculated and was incubated at 30℃ for 48 hr. Non-inoculated plates of CAS agar used as controls were incubated under the same conditions as described above. Orange halo zone formation against the blue background indicated siderophore production.
To investigate IAA production activity of isolates, strains were inoculated on King’s B (2% proteose peptone, 0.25% K2HPO4, 0.6% MgSO4, 1.5% glycerol) medium supplemented with 0.1% L-tryptophane at 30℃ under shaking for two days. After this, the medium of each culture was centrifuged at 13,000 rpm for 30 min, and the supernatant was mixed with Salkowski’s reagent (50 mL of 35% HClO4 and 1 mL of 0.5 M FeCl3) at a ratio of 1:2 (v/v); the mixture was then incubated at room temperature for 30 min [13]. Absorbance of samples was measured at 530 nm using a multifunctional microplate reader (SPARK 10M, Tecan Group Ltd., Männedorf, Switzerland). The method was similarly applied using IAA as a standard, and IAA concentrations in samples were obtained using a standard curve derived from the IAA standard solution [14].
Time course profiling of cell growth and antifungal activity
After sampling the bacterial culture of SRCM 121379, the absorbance was measured at a wavelength of 600 nm using glass-clear polystyrene (PS) cuvettes (ratiolab GmbH, Dreieich, Germany) with h=10 mm optical path length. Each cuvette was filled with 1 mL of bacterial suspension and the mean of 3 readings taken.
Optical density (OD) values and antifungal effect on C. gloeosporioides KACC 40003 was measured every four hours for two days.
Evaluation of plant growth promotion
In order to determine the effect of plant growth promotion of bacterial isolates, plant growth test was performed using Brassica juncea as a model plant. The isolates were incubated individually 100 mL flasks containing 30 mL LB broth medium at 30℃ for 24 hr and then cells were collected by centrifuging at 12,000 rpm for 5 min. Harvested cell pellets were washed twice with 0.1 M phosphate buffer solution (PBS, pH 7.0). Immediately before use, B. juncea seeds were incubated in 4℃ for 3 days beforehand to uniform germination and it was surface-sterilized with 70% EtOH for 5 min, 1% sodium chlorite (NaOCl) for 30 min, and rinsed in deionized water. Bacterial suspensions were treated in B. juncea seeds by soaking for 2 hr. For preparation control, seeds were soaked in deionized water.
Seeds (8 seeds per plate) were placed in Hoagland′s No. 2 Basal Salt Mixture (Sigma-Aldrich, St. Louis, USA) agar medium (0.5% Bacto-agar) and the agar plates were placed at 25℃ with an 18/6 light/dark cycle for 2 weeks. The plant experiment was performed by completely randomized block design with five replications. Plants were harvested two weeks after germination and lengths of shoot and root were measured. A one-way analysis of variance (ANOVA) was determined by Duncan’s multiple range test (DMRT). All statistical analysis was carried out using SAS 9.1 software (SAS Institute, Cary, NC, USA). Additionally, seedling vigor index was calculated as percentage of seed germination x (Mean shoot length+Mean root length).
Identification of Bacillus subtilisSRCM 121379
To identify the isolated bacteria, genomic DNA extraction and sequencing were conducted by Macrogen (Macrogen Inc., Seoul, Korea) using universal primers 785F (5´-GGATTAGATACCCTGGTA-3´) and 907R (5´-CCGTCAATTCMTTTRAGTTT-3´). Species identification was performed using the basic local alignment search tool (BLAST) algorithm on the NCBI server (http://blast.ncbi.nlm.nih.gov).
API 50 CHB and API ZYM profiles of Bacillus subtilisSRCM 121379
After the above experiments, B. subtilis SRCM 121379 was selected for the examination of the enzymatic activity (API 50 CHB and API ZYM). For the carbohydrate assimilation test, an API 50 CHB kit (Biomérieux, Marcy-L’Etoile, France) was used. B. subtilis SRCM 121379 was resuspended in API 50 CHB medium which recognizes the fermentation of 49 carbohydrates on the API 50 CHB strip. Tubes were filled with the inoculated medium and were covered using mineral oil, and strips were incubated for 24 and 48 hr at 30℃. When a carbon source is metabolized, the medium acidifies, and the red indicator in the medium changes to yellow. To test hydrolytic enzyme activity, an API ZYM kit was used. Strips were incubated for 4 hr at 37℃.
RESULTS AND DISCUSSION
Isolation of antifungal bacterial strains from soil samples
At present, chili pepper is grown around the world and is commonly used in many cuisines as a spice and in pharmacology for the obtaining of bioactive compounds called capsaicinoids [15]. Even though it is an important spice crop grown worldwide, many constraints decrease its production, causing significant reduction in yield and seed production. Plant diseases are the major cause of crop losses worldwide [1].
However, several strains of Bacillus sp. were reported to exert strong antifungal effects on different phytopathogens. It is important to select strains with high antifungal activity against fungal pathogens of chili pepper. In this study, field soil samples collected in Sunchang were used for isolation. Fifty strains of Bacillus were examined visually with the naked eye and were isolated.
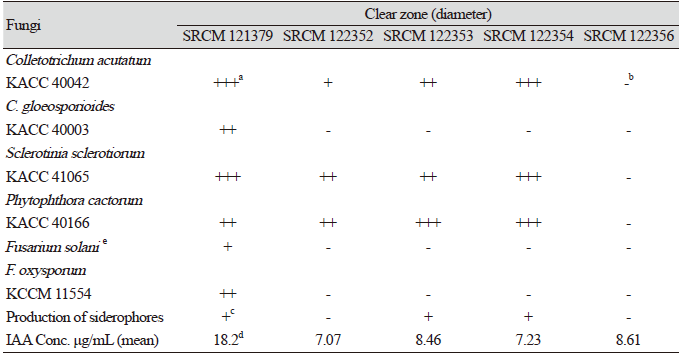
Table 1. Extracellular enzyme activities of the isolated strains.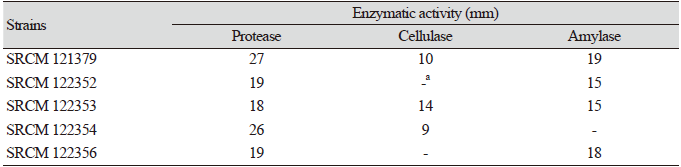
|
|
a No formation of clear zone or no activity |
Determination of microbial extracellular enzymes
Bacillus species are the main producers of extracellular proteases, and industrial areas frequently use B. subtilis for the production of diverse enzymes. Especially, proteases are known to be the major enzymes produced from microbial sources [16]. Therefore, the produced amounts of protease, cellulase, and amylase were assessed (Table 1). The size of the halo or clear zone formed around the well of each of the fifty strains was measured in order to quantify enzyme activity. Five bacterial isolates (strains SRCM 121379, SRCM 122352, SRCM 122353, SRCM 122354, and SRCM 122356) showed high activities of at least two types of enzymes, and these strains were screened first.
in vitro antifungal activities
The five selected strains were tested for their antifungal effects on six different phytopathogenic fungi by dual culture (Table 2; Fig. 1). After measuring the sizes of the clear zones, four of the five strains generally showed strong antifungal effects on C. acutatum KACC 40042, S. sclerotiorum KACC 41065, and P. cactorum KACC 40166. The results of the dual culture assay showed pathogen-specific effects by individual Bacillus strains. Strains SRCM 121379, SRCM 122353, and SRCM 122354 showed relatively strong inhibitory effects with inhibition zones of 10 mm or more.
Diseases caused by S. sclerotiorum are difficult to control because of the persistence of the pathogen in the soil for a longer duration of time and its production of air-borne ascospores. The most promising option to limit pesticide application would be the use of cultivars resistant to S. sclerotiorum [17]. C. acutatum, a pathogen of strawberry plants, also infects chili, as confirmed by numerous reports on the cross-infection ability of the different species of Colletotrichum originating from numerous hosts [18]. Plant breeders must consider the potential of C. acutatum to be a major pathogen when developing new chili cultivars with resistance to anthracnose disease [19]. Furthermore, P. capsici is the most notorious pathogen of chili, to which all parts of chili plant, at all developmental stages, are vulnerable. P. capsici is a general soil-borne pathogen, and it can invade healthy chili pepper fruit tissue and succulent stem tips [20].
In the current study, strain SRCM 121379 showed promising results regarding its use as a BCA, based on the observed antifungal effects. This result may indicate that Bacillus strain use different antagonistic mechanisms and produce different bioactive molecules to counteract different pathogens [21]. The consensus is that the application of biological methods appears safer for the environment than the use of artificial pesticides.
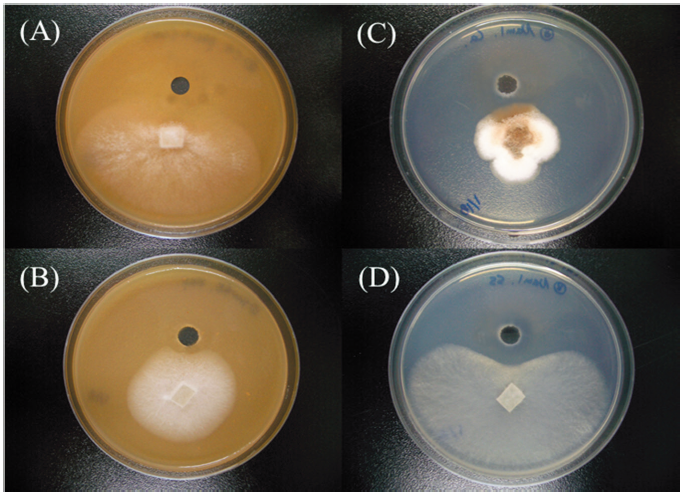
Fig. 1. Antifungal activities of Bacillus subtilisSRCM 121379 against several phytopathogenic fungal strains ([A-B], colonies on V8 juice agar and [C-D], potato dextrose agar) by dual culture. Bacillus subtilis SRCM 121379 against Phytophthora cactorum KACC 40166 (A), Fusarium solani which isolated from hydroponically cultured Panax ginseng (B), Colletotrichum acutatum KACC 40042 (C), and Sclerotinia sclerotiorum KACC 41065 (D) by agar well diffusion method
Plant growth promoting properties of bacterial isolates
Siderophores are compounds secreted under low-iron stress [22], and they are classified by the ligands used to chelate the ferric iron. These include catecholates, hydroxamates, and carboxylates [23]. Siderophores are important for the growth of many crop plants and increase their production by increasing the availability of Fe3+ to plants [24]. Coloration change from blue to orange results from siderophoral removal of Fe from the dye [22]. Three of the five strains presented a positive result in the CAS assay, which indicated that these microorganisms produced a siderophore (Table 2). It is expected that these three strains may be used as BCAs.
PGPR have the potential to synthesize auxins which support plant growth and development. Auxin-producing PGPR support plant growth even under stress and in the presence of inhibitory compounds by alleviating the adverse effects of the inhibitory compounds. Production of bacterial IAA can serve as an efficient plant growth-promoting attribute of PGPR to be considered for their effective application for plant growth enhancement [25].
Absorbance of five strains was measured at 530 nm. These bacterial isolates (strains SRCM 121379, SRCM 122352, SRCM 122353, SRCM 122354, and SRCM 122356) produced IAA to different extents, ranging from 7.07 to 18.2 μg/mL (Table 2). IAA concentrations were produced using a standard curve from an IAA solution. IAA production by Bacillus sp. isolated from the rhizosphere of corn plants was previously observed to range from 0.75 to 21.3 μg/mL [26]. Strain SRCM 121379 produced 18.2 μg/mL of IAA, thus appeared to be a promising IAA producer.
Time course profiling of cell growth and antifungal activity
The chili anthracnose disease caused by Colletotrichum species considerably reduces the quality and yield of chili pepper resulting in low returns to farmers. Especially, C. gloeosporioides infects both in young and mature chili pepper at all developmental stages [3]. Therefore, in order to investigate the antifungal activity according to the cell growth, cell growth and antifungal activity against C. gloeosporioides KACC 40003 of strain SRCM 121379 was measured every four hours for time course profiling. To investigate the optimal cell growth of SRCM 121379, seed culture was inoculated on LB medium. OD values showed growth started to increase from the 4 hr, and showed the best growth at 24 hr. Also, antifungal activity against C. gloeosporioides KACC 40003 was high 24 hr after and stabilized (Fig. 2). Therefore, the optimal incubation time of SRCM 121379 is determined from log phase to stationary phase. This result is similar to B. subtilis showing highest total yield of cells from 24 hr [27]. Further research is needed for development antifungal agents using SRCM 121379.
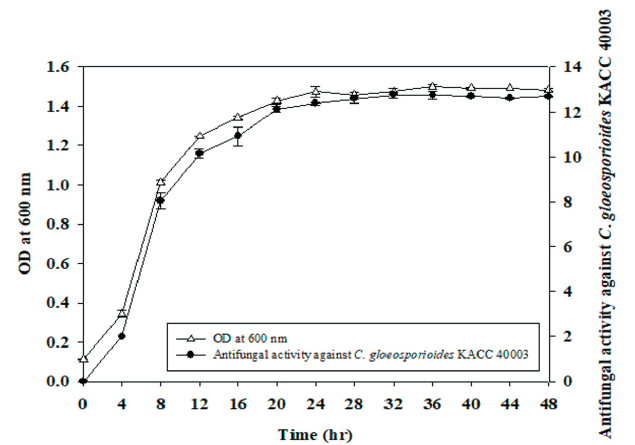
Fig. 2. Brassica juncea has a relatively short life cycle of 140-160 days [28]. This plant is used as a model The time course profile for cell growth of SRCM 121379. Time course profiles of optical density (OD) and antifungal activity against Colletotrichum gloeosporioides KACC 40003 for SRCM 121379 cultivated in potato dextrose agar (PDA) medium.
Growth of Brassica juncea
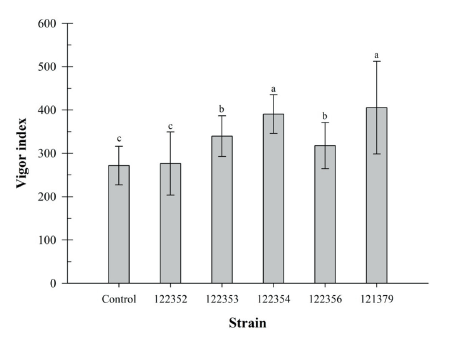
Brassica juncea has a relatively short life cycle of 140-160 days [28]. This plant is used as a model plant for plant growth promotion. The influence of bacteria inoculation on the seed germination rate and plant growth promotion using B. juncea were studied (Fig. 3). Germination rate of B. juncea seeds was determined as 95% and it was not affected by bacteria inoculation. However, shoot and root length of B. juncea were significantly increased by inoculation of SRCM 122354 and SRCM 121379 strain (Fig. 3A and 3B), respectively. Especially, great growth promoting effect on the shoot length elongation was observed in SRCM 121379 strain (45.6%) and root length elongation was observed in SRCM 122354 (96.0%). Furthermore, significantly highest vigor indexes also were observed in SRCM 122354 (390.4) and SRCM 121379 (405.5) when compared to control (271.6) (Fig. 4). These results suggest that SRCM 122354 and SRCM 121379 has superior plant growth promoting properties. In previous studies suggest that when plants were inoculated with PGPR, the extent of shoot and root elongation showed greater plant growth response than without inoculated plants [29].
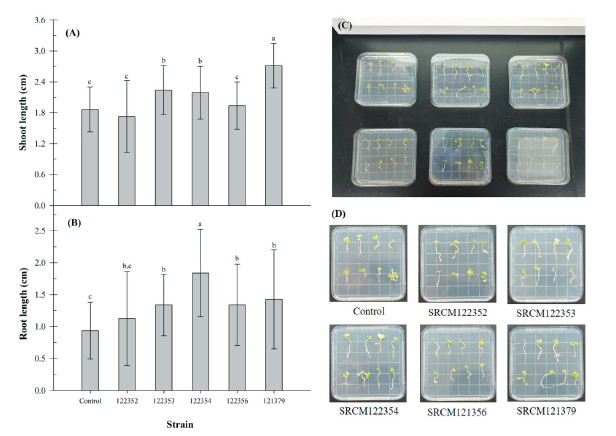
Fig. 3. Effect of inoculation with bacterial strains on Brassica juncea. (A), shoot length (B), root length (C-D), shoot and root regeneration from Brassica juncea. Bars represent standard deviations. a-c: means followed by the same letter are not different according to the Duncan’s multiple range test (p<0.0001,n=8).
Identification of Bacillus subtilisstrain SRCM 121379
The 16S rDNA sequence analysis result showed that the selected strain with antifungal effects against phytopathogenic fungi of chili pepper was Bacillus subtilis(99.59% identity), which was designated Bacillus subtilisstrain SRCM 121379. The sequence of B. subtilis strain SRCM 121379 was aligned using BioEdit Sequence Alignment Editor 7.2.5 software, and a phylogenetic tree was produced with the neighbor-joining method (1,000 bootstrap replications) using Molecular Evolutionary Genetics Analysis 10.1.8 software (Fig. 5). Phylogenetic analysis and NCBI BLAST results indicated that strain SRCM 121379 was closely related to other B. subtilis species. The B. subtilis strain SRCM 121379 was deposited in the Microbial Institute for Fermentation Industry, Korea, under the accession number SRCM 121379.
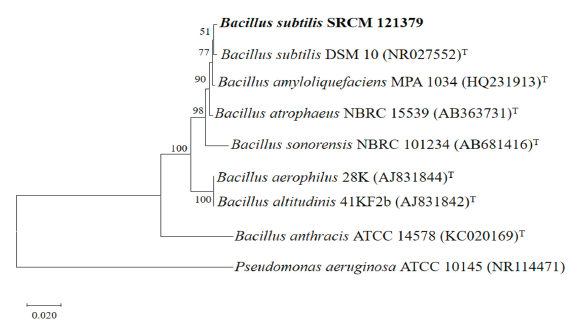
Fig. 5. Phylogenetic tree based on the Neighbor-joining (NJ) method analysis of 16S region of rRNA for Bacillus subtilisSRCM 121379. Numbers at the nodes indicate the bootstrap values (>50%) from 1,000 replications. The strain isolated in this study is shown in boldface. Pseudomonas aeruginosa was used as an outgroup. Bar, 0.020 substitutions per nucleotide position. T, type species.
Table 3. Carbohydrate assimilation of Bacillus subtilisSRCM 121379 assessed using anA PI 50 CHB kit.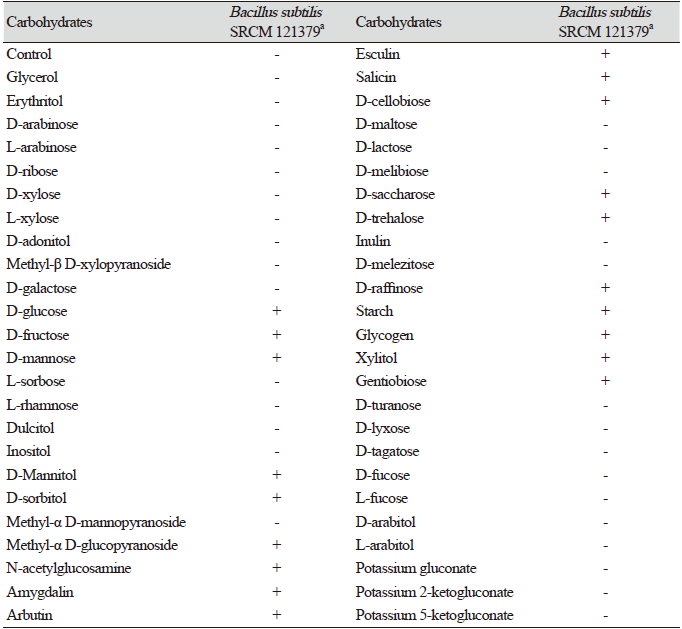
|
|
a+, utilized; -, not utilized. |
Table 4. Enzymatic activities ofB acillus subtilis SRCM 121379 assessed using anA PI ZYM kit.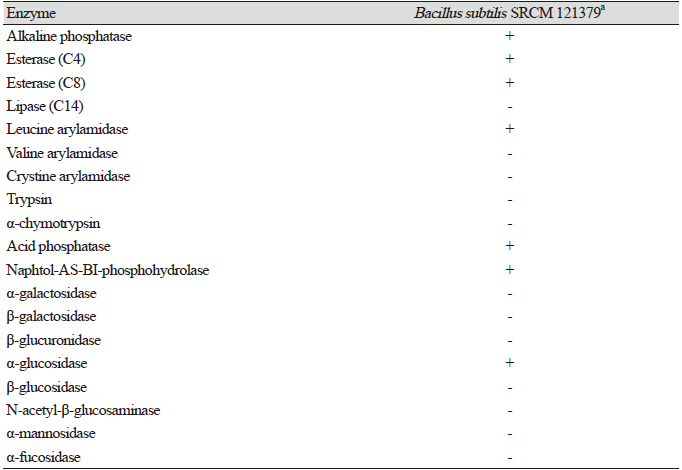
|
|
a+, utilized; -, not utilized. |
Profiles of carbohydrate assimilation and enzymatic activities for Bacillus subtilisSRCM 121379
B. subtilis SRCM 121379 was selected for assessing enzymatic activity (API 50 CHB and API ZYM). The API 50 CHB test was used to analyze bacterial growth on different carbohydrates and their derivatives (heterosides, polyalcohols, and uronic acids). During the incubation of the API strips, fermentation was visualized through a color change in the tube, caused by the production of acid and recognized by a pH indicator in the medium. B. subtilis SRCM 121379 showed positive reactions with 19 carbohydrates including glucose, fructose, mannose, mannitol, and sorbitol; however, negative reactions with 30 carbohydrates including rhamnose, fucose, and tagatose were observed (Table 3). Using an API ZYM kit, B. subtilis SRCM 121379 showed positive enzymatic activity with esterase C4, esterase C8, α-glucosidase, and naphtol-AS-BI-phosphohydrolase, among others (Table 4). Especially, esterase plays crucial role in the degradation of wide variety of pollutants in the environment. Esterase belongs to hydrolase group of enzymes and found capable of hydrolyzing a large number of ester linkage and ester bond containing compounds [30].
In conclusion, the amounts of protease produced by B. subtilis SRCM 121379 were higher than other strains. Considering their wide application in various industries, protease enzymes occupy an important position [16]. Results of in vitro antifungal activities showed B. subtilis SRCM 121379 has antifungal activities against various phytopathogens. Especially, B. subtilis SRCM 121379 controlled Colletotrichum species which generally cause anthracnose in chili pepper. On the other hand, shoot and root length of B. juncea were notably increased by inoculation of SRCM 121379 (Fig. 3A and 3B). Not only based on identification of the strain SRCM 121379 using 16S rRNA gene sequences analysis, but also API 50 CHB and API ZYM tests showed general features of B. subtilis [31]. Based on overall results, B. subtilis SRCM 121379 showed sufficient potential to be utilized as BCA and PGPR. In the future, if field experiments and mass production process development as a potential BCA are successfully added, the industrial value of B. subtilis SRCM 121379 will be more improved.