Pyogo (Lentinula edodes) is a basidiomycete of the order Agaricales that causes white rot in dead hardwoods [1]. This mushroom is rich in nutrients and a good source of provitamin D2. Pyogo is also known for its high pharmaceutical value, owing to the presence of polysaccharides and many compounds with antiviral, anticancer, antioxidant, and immunomodulatory activities [2-4]. Because of these positive attributes, pyogo is one of the most cultivated edible mushrooms, accounting for approximately 22% of global mushroom production, and is commercially cultivated in many countries, including by major producers in Korea, China, Japan, Australia, the United States, and Canada [5,6]. In 2020, pyogo production in Korea was approximately 20,207 tons, making it one of the most important forest products, accounting for approximately 98% of total forest mushroom production [7]. Because pyogo has a high economic value, individual breeders, the seed industry, and national institutions are developing new cultivars [8].
Plant variety protection is a system that protects new cultivars from unauthorized use and encourages breeding [9]. A specific cultivation test is required to confirm the differentiation, uniformity, and stability of cultivars for registration and protection. This cultivation test is a time-consuming, labor-intensive process because it is mainly based on morphological characteristics and is vulnerable to environmental factors [10,11]. For these reasons, the International Union for the Protection of New Cultivars (UPOV) suggests using sequence-based markers, such as reproducible simple sequence repeats (SSR), single nucleotide polymorphisms (SNP), and cleavage amplification polymorphisms (CAPS) [12,13].
Single nucleotide polymorphisms (SNPs) are mutations in a single nucleotide sequence that are caused by a transition between purines (A, G) or pyrimidines (C, T). SNP is the most widely used molecular marker because of its broad-spectrum distribution and lower analysis cost than other markers [14-16]. SNP discovery via Sanger sequencing technology is based on PCR and has low throughput. However, with the development of next-generation sequencing (NGS), high-throughput SNPs in short time navigation became possible [17]. Electrophoresis has been used in traditional SNP genotyping methods, such as cleaved amplified polymorphic sequence (CAPS), dCAPs (derived CAPS), and AS-PCR (allele-specific PCR) [18]. Because of technical advances, high-resolution melting (HRM)-based SNP marker analysis can efficiently use a fluorescent dye interposed between DNA double strands to measure the rate of DNA dissociation from double to single strands. It has been reported as one of the most powerful methods for analyzing genetic variation [19-21]. HRM analysis has been successfully used to identify crop cultivars, such as cherries, tangerines, peppers, and pomelo, as well as to ensure the authenticity of olive oil and wine [22-26]. In the case of mushrooms, HRM analysis has been used to identify hallucinogenic mushrooms and enoki mushrooms, as well as the pyogo cultivar Gyunheung No. 115 [27-29].
The most popular pyogo mushrooms cultivated on sawdust medium in Korea are Sanjo 701 and Chamaram, two new cultivars developed in the Federation Forest Mushroom Research Center of Korea. In 2020, the preference for the two cultivars in Korea was 45.2 percent and 25.2 percent, respectively [30]. Sanjo 701 is characterized by its bright color, firm flesh, and heft. Chamaram has a quantitative trait similar to that of Sanjo 701, with the additional advantage of being easily diversified in commercial distribution because of its excellent growth properties over a wide temperature spectrum [30]. However, no scientific or technical approach has been developed to adequately protect these two important cultivars from illegal distribution in domestic and global markets. In this study, we developed SNP markers that differentiated Chamaram and Sanjo 701 from 23 commercialized pyogo cultivars and then applied HRM analysis to secure a scientific method for distinguishing the two major pyogo cultivars.
For developing SNP markers that differentiate Sanjo 701 and Chamaram from one another, 22 locally developed strains and one imported strain were used [31], with B17 serving as a standard whole genome of L. edodes (Table 1). Mycelia of the tested strains were cultivated in dark culture at 25℃ in a medium containing potato dextrose agar.
Table 1. List of strains analyzed in this study.
|
Genomic DNA was extracted from mycelium cultured by shaking at 25℃ and 110 rpm under dark conditions for approximately 2 weeks. The cultured mycelium was washed with PBS buffer (NaCl 135 mM, KCl 2.7 mM, Na2HPO4 4.3 mM, KH2PO4 1.4 mM) and dried with a kitchen towel. Approximately 100 mg of dried mycelium was frozen in liquid nitrogen and finely ground with a mortar, and genomic DNA was extracted using the GenEx™ Plant kit (GeneAll, Seoul, Korea). The extracted DNA was quantified with a micro-spectrophotometer K5600 (BioFuture Inc., China), diluted to 20 ng/μL and used in this study.
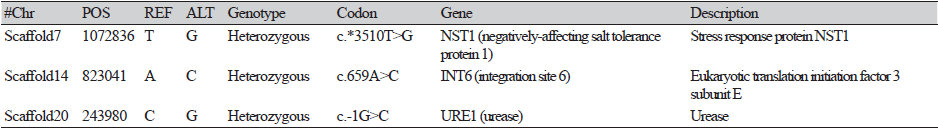
The method developed by Hillier et al. [32] was used to identify SNPs found in Sanjo 701 and Chamaram. Genomic DNA extracted from 23 cultivars was resequenced on the HiSeq 2500 platform, and SNPs found specifically in the genomes of Sanjo 701 and Chamaram were selected (Tables 2 and 3). One homozygous SNP, three heterozygous SNPs from Sanjo 701, and three heterozygous SNPs from Chamaram were chosen because only missense SNPs in the coding region of specific genes were selected (Tables 2 and 3).
Primer Express software (Applied Biosystems, Foster City, CA, USA) was used to extract the flanking sequence from the selected SNP and amplify the marker sequence. PCR amplification of the marker regions was performed by 35 cycles at 95℃ for 3 min, 95℃ for 30 s, 55℃ for 30 s, and 72℃ for 20 s, followed by a 5 min reaction at 72℃.
PCR for HRM analysis was performed using 20 ng genomic DNA 1 μL, 2X MeltDoctor™ HRM Master Mix (Applied Biosystems, Foster City, CA, USA), 10 μL 10 pmole/μL primers F and R (1 μL each), and 7 μL DW. After denaturation at 95℃ for 10 min using QuantStudio 3 (Applied Biosystems, Foster City, CA, USA), denaturation at 95℃ for 15 s, annealing at 54℃ for 30 s, and extension at 72℃ for 30 s were performed on a mixture of 20 μL. After 40 repetitions and 10 s of heating at 95℃, the melt curve was determined by gradually increasing the temperature from 60℃ to 95℃ at a rate of 0.025℃/s. High-resolution melt software version 3.2 was used to analyze the melting curve (Applied Biosystems, Foster City, CA, USA).

Fig. 2.Discrimination of Sanjo701 and Chamaram by high-resolution melting curve analysis among 23 pyogo cultivars. Aligned melting curve analysis (A) and differential plot (B) of RLLE- 002 using ‘Sanjo101’ as a reference. Aligned melting curve analysis (C) and differential plot (D) of RL-LE-203 using ‘Chunjang2’ as a reference. Results for Sanjo701 and Chamaram are in blue, and those for the other 22 strains are in red.
Using Sanger sequencing, we confirmed the SNPs on 4,123,084 bp in Scaffold 7 in the genome of Sanjo 701, which changed the nucleotide sequence from G to A (Fig.1A; Table 2), and 823,041bp in Scaffold 14, which changed the nucleotide sequence from A to C in the Chamaram genome (Fig.1B; Table 3). However, Sanger sequencing further proved that additional non-specific SNPs existed in genomic regions other than the selected SNPs (highlighted in red in Tables 2 and 3). On the basis of these results, two specific SNPs, Chamaram and Sanjo 701, were selected as the most suitable targets for HRM marker development. The RL-LE-202 and RL-LE-203 primer sets were designed to produce 104 and 112 bp amplicons for each SNP-containing sequence, respectively, by PCR. (Table 4).
HRM-PCR analysis of 23 strains, including Sanjo 701 and Chamaram, revealed that the RL-LE-202 marker clearly produced a heterotype curve for Sanjo 701 and homotype curves for the other strains (Figs. 2A and B). Chamaram was also distinguished by a specific heterotype curve by using the RL-LE-203 primer set (Figs. 2C and D). These findings indicate that the cultivars Sanjo 701 and Chamaram can be precisely differentiated using the SNP-based HRM markers developed in this study.
SNP and indels are the two most common types of nucleotide sequence polymorphism. Most of them are biallelic and found in large quantities across the genome [33]. SNPs found in the coding area of genes can be employed more effectively than randomly selected SNPs to discover functionally significant variations [34]. However, because the amount of genetic information per marker is modest, the duration of the marker development selection process is long and is restricted because it needs to evaluate many loci [35,36]. NGS technology delivers large-scale sequence datasets that can be used for genetic mapping, genetic diversity analysis, gene identification, and molecular breeding. These large-scale sequence datasets can also be used to define sequence diversity and create polymorphism and genotyping data [37-39]. Other applications of this technology include digital molecular breeding. This instrument is excellent for performing such tasks [40]. Additionally, it allows for the discovery of new or reference-based genomic variations, even in organisms with little or no genetic information, and provides a quick, easy method to obtain the location and genotype information of the related genome [41,42].
This study demonstrates that SNP identification, marker development, and HRM analysis can be successfully applied to the discrimination of pyogo cultivars with low genetic diversity by using comparative genomics with the whole genomic information of the B17 L. edodes strain. The developed SNP marker can accurately identify shiitake varieties even at the mycelial growth stage; thus, we expect it to prevent the mixing of varieties. In addition, the SNP marker development method presented in this study can be used to develop markers that can discriminate among other edible mushrooms.