Introduction
Truffles are fungi belonging to the genus Tuber that produce hypogeous fruiting bodies with a unique taste and aroma. They have ectomycorrhizal (ECM) association with several host plants, especially oak and hazel trees, by colonizing the cortex cells of their roots to form a Hartig net and creating a thick mycelium outside the roots [1]. Tuber spp. have high host specificity [2]. For example, Tuber melanosporum shows a higher host preference for Northern Hemisphere angiosperms (e.g., Quercus spp., Corylus spp.) but a lower host preference for Pinus spp. In contrast, Tuber borchii has a wider host range, including angiosperms (e.g., Quercus spp., Populus spp.) and gymnosperms (e.g., Pinus spp., Picea spp., Cedrus spp.) [3]. The selection of host plants affects mycorrhiza formation [4], and the rate of mycorrhizal root colonization varies depending on the host plant [5]. The Tuber ECM, such as the ECM system or parenchymatous mantle structure, has general characteristics [6]. However, there are distinctive features of the ECM according to the host plant and Tuber spp. For example, the Tuber indicum ECM shows morphological differences depending on the host plant [7,8]. In contrast, the mycorrhizal characteristics of Chinese white truffles on the same host plant can be different [9].
In this study, we inoculated a spore suspension of European truffles, T. borchii and T. melanosporum, onto an indigenous host plant, Quercus acutissima. The Périgord black truffle (T. melanosporum) and T. borchii are European truffles of high economic and commercial value. Furthermore, these are the most successfully cultivated species in many country [10-12]. However, there has been no record of successful mycorrhization between those truffles with host plants in Korea, although T. himalayense and T. huidongense collected in Korea were found to produce mycorrhiza with two oak trees in Korea [5]. Moreover, Q. acutissima is a widely distributed oak species in Korea. Therefore, the objectives of this study were (1) to determine whether European truffle species can form ECM with an indigenous host plant and (2) to identify the morphological and anatomical characteristics of truffle mycorrhizae.
Materials and Methods
Preparation of seedlings
Q. acutissima seeds were collected from a natural site in Cheongju, Korea. The shells of these seeds were then removed. The seeds were washed with tap water and surface sterilized with 10% sodium hypochlorite (NaOCl) for 30 min. For germination, seeds were placed in plastic pots (280 mL) with a mixture of sterile vermiculite and perlite (1:1 ratio) and maintained in a growth room for 6 months (8 h photoperiod per day, 55±5% relative humidity, and 24±1℃ temperature).
Inoculation onto seedlings and mycorrhizal synthesis
Ascocarps of T. borchii and T. melanosporum were purchased from Chiko Corporation, Korea. The ascocarps were rinsed with tap water, sterilized with 70% ethanol, and then ground in sterile water. Six-month-seedlings of Q. acutissima were inoculated with 1 mL of a spore suspension containing 1.4×10℃ spores/mL, which were counted using a hemocytometer (Paul Marienfeld GmbH & CO., KG, Lauda-Königshofen, Germany). The inoculated seedlings were placed in plastic pots (280 mL) with a mixture of sterile vermiculite and perlite (1:1 ratio). They were cultured in a growth room for 6 months and watered weekly (8 h photoperiod per day, 55±5% relative humidity, and 24±1℃ temperature).
Molecular identification of ECM
The mycorrhizal root tips of inoculated seedlings were sampled to confirm mycorrhiza formation after 2 months of inoculation. Genomic DNA was extracted using the HiGene™ Genomic DNA Prep Kit (BIOFACT, Daejeon, Korea). The rDNA region, including the internal transcribed spacer (ITS) region, was amplified by PCR using ITS1F and ITS4 primers [13]. Nucleotide sequences were analyzed at Solgent (SolGent Co., Ltd., Daejeon, Korea). Sequence similarity was determined using BLAST (https://www.ncbi.nlm.nih.gov/).
Six months after inoculation, the morphological characteristics of T. borchii and T. melanosporum ECM were observed. First, the ECM of inoculated seedlings was sampled. Then, the morphological characteristics of the ECM were observed using a dissection microscope (Olympus SZX7, Tokyo, Japan). Cross-and longitudinal sections were obtained using a cryostat (CM1850, Leica, Wetzlar, Germany). Subsequently, anatomical characteristics were observed via a light microscope (Axio Imager A1, Carl ZEISS, Oberkochen, Germany).
Results
Mycorrhization and molecular identification of ECM
Mycorrhization of T. borchii and T. melanosporum on Q. acutissima was confirmed 2 months after inoculation. The ITS sequences amplified from the mycorrhizal root tips of T. borchii (NCBI accession No. OP975718) and T. melanosporum (NCBI accession No. OP978675) showed more than 99% similarity with those of sporocarps inoculated to seedlings using BLAST.
Morphological and anatomical characterization of ECM
Six months after inoculation, 20 seedlings that formed mycorrhiza were selected per Tuber species. Morphological and anatomical features of the ECM of T. borchii and T. melanosporum were observed, measured, and analyzed (Table 1). For the T. borchii ECM, the ectomycorrhizal system was simple, with a monopodial pinnate pattern. The unramified ends were straight, cylindrical, or club-shaped with rounded ends. Their texture was short-spiny, and their color was whitish light brown to brown and rarely dark brown (Fig. 1). The mantle type had a non-interlocking pattern, with an angular pattern arranged in 2-5 hyphal layers. The outer mantle was yellowish and reddish brown. The emanating hyphae were straight, simple, bristle-like, awl-shaped, and septate.
Table 1. Dimensional measurements of Tuber borchii and Tuber melanosporum mycorrhizae (mean±standard error).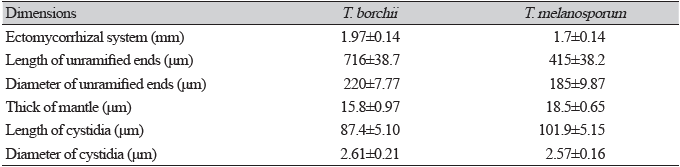
|
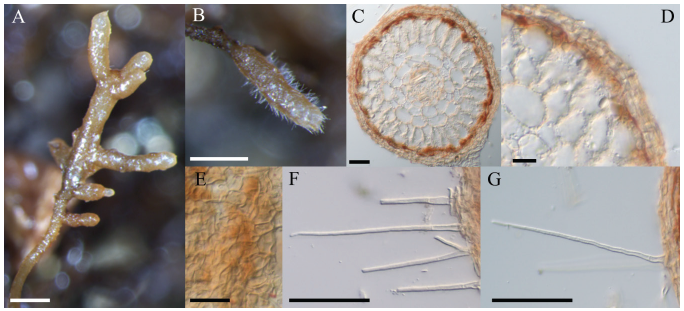
Fig. 1. Macro-morphological and anatomical characteristics of ectomycorrhizal roots of Quercus acutissima inoculated with T. borchii. Mycorrhizal root tips (A, B); cross-section of mycorrhizal root tips (C, D); outer mantle surface structure (E); and separate hyphae emanating from outer mantle layer (F, G) (scale bars: A, B=500 μm; C-E=20 μm; F, G=50 μm).
ECM of T. melanosporum was simple and rarely followed a monopodial pinnate pattern (Fig. 2). The shape of the unramified ends was straight, bent, cylindrical, or mostly club-shaped with rounded ends and often long-spiny. Their colors were ochre, brown, dark brown, and mostly black. The mantle type showed an irregular interlocking pattern with 2-5 angularly arranged hyphal layers. The outer mantle was reddish-brown and brown. The emanating hyphae were bent, simple, bristle-like, awl-shaped, and septate and had right-angle ramifications.

Fig. 1. Macro-morphological and anatomical characteristics of ectomycorrhizal roots of Quercus acutissima inoculated with Tuber melanosporum. Mycorrhizal root tips (A, B); cross-section of mycorrhizal root tips (C, D); outer mantle surface structure (E); and separate hyphae emanating from outer mantle layer (F, G) (scale bars: A, B=500 μm; C-E=20 μm; F, G=50 μm).
Discussion
The time required for mycorrhiza formation differs depending on the host species [5]. T. borchii and T. melanosporum ECM on Q. acutissima exhibited mycorrhization 2 months after inoculation. In previous studies, the mycorrhization of T. borchii with Arbutus unedo was found to occur 2 months after inoculation [14] and that of T. melanosporum with Quercus spp. was observed 6 months after inoculation [10]. In Tuber himalayense and T. huidongense, mycorrhization with an indigenous Quercus spp. requires 2.5 months, and it was confirmed that mycorrhization occurs more quickly compared to that with other host plants [5]. These results indicated that Tuber spp. have a higher preference for native oak trees in Korea. Moreover, Q. acutissima was found to be a suitable host plant for Tuber spp.
Compared to those reported in previous studies, most ECM features of T. borchii and T. melanosporum have been described similarly. However, distinct features were also observed. The T. melanosporum ECM with Quercus spp., A. unedo, and Carya illinoinensis is yellow-ocher or brown [10,15,16], but that with Q. acutissima is mainly dark. In terms of the mantle structure, the T. borchii ECM with A. unedo, Tilia platyphyllos, C. illinoinensis, and Quercues robur has an irregular interlocking pattern [14,17-19], whereas that with Q. acutissima shows a non-interlocking irregular pattern.
Despite the host plant being the same, the characteristics of mycorrhizae vary depending on the Tuber species. In this study, the ECM and mantle color of T. borchii were brighter than those of T. melansoporum. In the mantle type, T. borchii showed a non-interlocking irregular pattern, whereas T. melanosporum showed an irregular interlocking pattern. Whereas T. borchii had straight cystidia, T. melanosporum had bent cystidia. The mycorrhizal characteristics of the two Tuber spp. were thus clearly different. It is reasonable to combine morphological and anatomical characteristics to distinguish the ECM of different Tuber species [6]. However, these characteristics alone are not sufficient to determine which Tuber species form mycorrhizae. This is because phylogenetically similar Tuber species also have similar mycorrhizal characteristics; therefore, molecular analysis is needed for accurate identification [8].
We successfully induced mycorrhization between a European truffle species and an indigenous oak species in Korea. The inoculation method using a spore suspension could further be applied to the mycorrhization of European truffle species on indigenous oak species in Korea. This suggests that indigenous oak can be an appropriate host plant for commercial truffle cultivation. In addition, mycorrhiza formation by Tuber spp. with other indigenous oak species needs to be confirmed. This is the first report of the mycorrhization of T. borchii and T. melanosporum with Q. acutissima. Thus, the range of host plants for T. borchii and T. melanosporum has expanded. This study provides the morphological and anatomical characteristics of the ECM depending on the Tuber spp. However, further studies are needed to determine whether the mycorrhizae of Tuber spp. are maintained in a natural environment after transplant.


