INTRODUCTION
Talaromyces was introduced by Benjamin [1] to include species of certain penicilla-producing teleomorphic gymnothecial ascocarps, and Talaromyces vermiculatus was the first species described that presented soft cottony-fruiting bodies and cell walls made from tightly interwoven hyphae. Talaromyces belongs to the family Trichocomaceae, order Eurotiales of the kingdom Ascomycota, which comprises 64,000 fungal species [2]. Certain species of Talaromyces, including T. verruculosus, T. aculeatus, and T. apiculatus, are the only species of Talaromyces that produce conidiophores with ampulliform phialides, which taper into very thin necks and have rough-walled, globose conidia [2]. Previous phylogenetic analysis revealed that Talaromyces and members of the Penicillium subgenus Biverticillium belonged to a clade peculiar to Penicillium sensu stricto [3-4]. Subsequently, following the concept of single-name nomenclature [5], all accepted species of the Penicillium subgenus Biverticillium were transferred to Talaromyces. Based on morphology and multigene phylogeny, Yilmaz et al. [6] studied the taxonomy of Talaromyces and 88 species were categorized into seven sections: Talaromyces, Bacillispori, Helici, Islandici, Purpurei, Subinflati, and Trachyspermi.
In 2017, during the study and survey of fungal species in the field soil of Yangpyeong, South Korea, several fungal species were isolated and identified. Among the identified species, Talaromyces veerkampii was also recovered. In Korea, this Talaromyces species was recovered for the first time from field soil. Thus, we report this here as a new official record. The primary focus of this study was to (i) examine the T. veerkampii isolate at the morphological and molecular level and (ii) compare this T. veerkampii isolate with those of previously recorded T. veerkampii.
MATERIALS AND METHODS
Soil sample collection and fungal isolation
Soil samples were collected from various field locations in Yangpyeong in 2017. The global positioning system coordinates of the soil sampling location were 37°28′52.81″N and 127°36′12.26″E. Soil samples were collected from depths of 0 to 15 cm. Surface leaf litter and plant debris were discarded, and the soil was stored in plastic zipper bags and transported to the laboratory. The collected soil samples were dried at room temperature, ground, and sieved using an autoclave – sterilized brass sieve with an aperture size of 2 mm. Soil samples were serially diluted using a conventional dilution plating technique to separate fungal isolates [7]. Briefly, 1 g of soil sample was suspended in 9 mL of sterile distilled water and mixed using a vortex machine for 2–3 min. The suspended soil samples were serially diluted in sterile distilled water. One milliliter of each 10-3, 10-4, 10-5, and 10-6 dilution was pipetted out and poured into 90 mm Petri plates containing potato dextrose agar (PDA; Difco Laboratories, Detroit, MI, USA) supplemented with 100 mg/L chloramphenicol (a bacteriostatic agent). Petri dishes were then incubated at 25℃ for 7 days until fungal colonies were visually observed. Distinct morphological colonies were selected and purified on freshly prepared Petri plates containing PDA medium. The freshly prepared pure culture of the fungal isolate was preserved in 20% glycerol stock at 4℃ for further studies.
Morphological characterization
Morphological characterization of the fungal isolate KNU17-180 was performed based on the growth patterns on PDA (MB Cell, Los Angeles, CA, USA). The strain was inoculated at one point at the center of a freshly prepared 9 cm diameter PDA plate. The inoculated plates were incubated for 7 days at 25℃ in the dark. The media were prepared following the method described by Samson et al. [8]. After incubation, the colony diameter of the isolate KNU17-180 was observed on PDA medium. In addition, morphological characteristics such as colony color (obverse and reverse sides), colony diameter, and degree of sporulation, margin shape, elevation, and shape and size of micromorphological characteristics were also observed. Colony photomicrographs of the isolates were evaluated using a 3.1 CMOS digital camera (KOPTIC Korea Optics, Seoul, Korea) attached to an Olympus BX50F-3 microscope (Olympus Optical Co., Ltd, Tokyo, Japan). Micromorphological attributes of the isolate were also observed using a Leo Model 1450VP variable pressure scanning electron microscope (Carl Zeiss, Cambridge, Massachusetts, USA).
Genomic DNA extraction, PCR amplification, sequencing, and phylogenetic analysis
Genomic DNA of the fungal isolate was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. The internal transcribed spacer (ITS) region and calmodulin (CaM) gene were amplified using the primers ITS1 (5′- TCCGTAGGTGAACCTGCG-3′) and ITS4 (5′- TCCTCCGCTTATTGATATGC-3′) [9] and CMD5 (5′- CCG AGT ACA AGG CCT TC-3′) and CMD6 (5′- CCG ATA GAG GTC ATA ACG TGG-3′) [10], respectively. Amplified PCR products were sequenced using an ABI Prism 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA). The conditions used for the thermal cycles for amplification and sequencing are presented in Tables 1 and 2, respectively. Reference sequences for ITS and CaM of the study isolate were compared with those in the GenBank database at the National Center for Biotechnology Information (NCBI) applying the basic local alignment search tool (BLAST) [11]. After confirming the study isolate through NCBI BLAST analysis, the nucleotide sequence was deposited at the culture collection of the National Institute of Biological Resources (NIBR, Incheon, Korea). The culture deposition number for isolate KNU17-180 was assigned as NIBRFGC000500019 in the NIBR database. Furthermore, the nucleotide sequence of isolate Talaromyces veerkampii KNU17-180 was deposited in the GenBank database of NCBI and assigned the accession number MH231769. The nucleotide sequences generated from the study isolate were edited, merged, and proofread for comparison with the sequences retrieved from the GenBank database using MEGAX [12]. A neighbor-joining phylogenetic tree was constructed using the Kimura 2-parameter substitution model [13], and bootstrap analysis was performed with 1,000 replications to determine the support for each clade.
RESULTS
Talaromyces veerkampii:
Talaromyces veerkampii C.M. Visagie, N. Yilmaz, & R.A. Samson, Mycoscience (2015) [1] [MB#808233].
Morphology of the isolate KNU17-180
Colony morphology
The detailed morphology of the isolated colony, including the microstructures, is shown in Fig. 1. On PDA medium, the obverse side of the colony was white in color. The reverse side of the colony was white with red spots. (Fig. 1A and 1B). The colony grew rapidly and attained a diameter of 55–60 mm after 7 days at 25℃. The texture of the colonies was floccose. The surface was smooth. Conidia were in a mass. The elevation was flat, and the margin was entire. A circular form and exudates were absent.
Micromorphology of the isolate KNU17-180
The detailed microstructures of the isolate are shown in Fig. 1. A brief description and comparison of the isolates are presented in Table 3. Conidiophores were biverticillate (Fig. 1C), tapering into a very thin neck (Fig. 1D) producing rough-walled globose conidia (Fig. 1E and 1F) that were 37–108 × 2.8–3.1 μm in size. Metulae were divergent; 4–8, 7.5–12.8 × 2.6–3.4 μm in size (Fig. 1G). The phialides were ampuliform and tapered into a very thin neck; 8.2–11.1 × 3.1–3.6 μm in size (Fig. 1H). Conidia were broadly ellipsoidal and finely rough-walled (Fig. 1I) and were 4–4.7 × 2.9–3.8 μm in size.
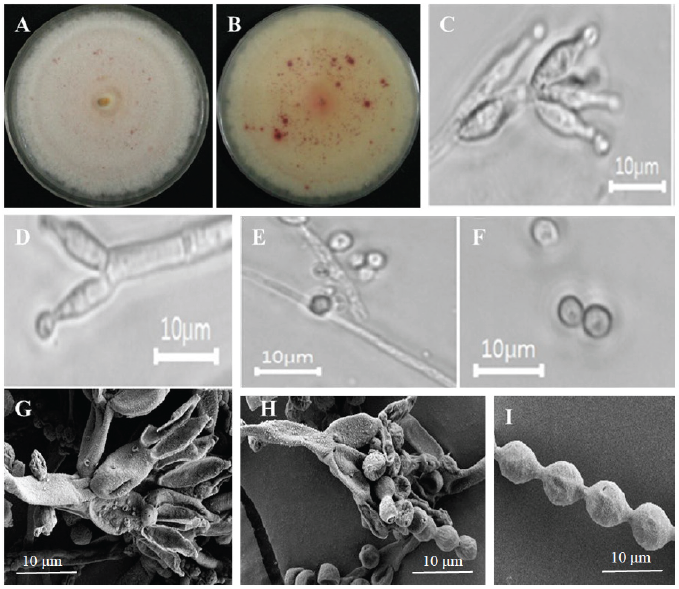
Fig. 1. Morphological characteristics of Talaromyces veerkampii KNU17-180 grown on potato dextrose agar (PDA) at 25℃ for 7 days. (A) Obverse of colony, (B) reverse of colony, (C-F) simple microscopy images of conidiophores and conidia, (G,H) scanning electron microscopy images showing phialides and metulae, (I) scanning electron microscopy images showing conidia.
Molecular phylogeny of the isolate KNU17-180
The ITS region and CaM sequences were obtained from NCBI BLAST analysis to examine the close relationships between the isolate KNU17-180 and a previously described reference species. The 18S-ITS1-5.8S-ITS2-28S rDNA sequences revealed that isolate KNU17-180 was the most closely related to Talaromyces veerkampii (NR153228.1) with a bootstrap value of 95% (Fig. 2). In addition, 98% similarity was obtained between the CaM sequence of isolate KNU17-180 and that of the reference isolate from the NCBI BLAST database (KF741961.1) (Fig. 3). These results indicate that our study isolate KNU17-180 is 98% identical to T. veerkampii.
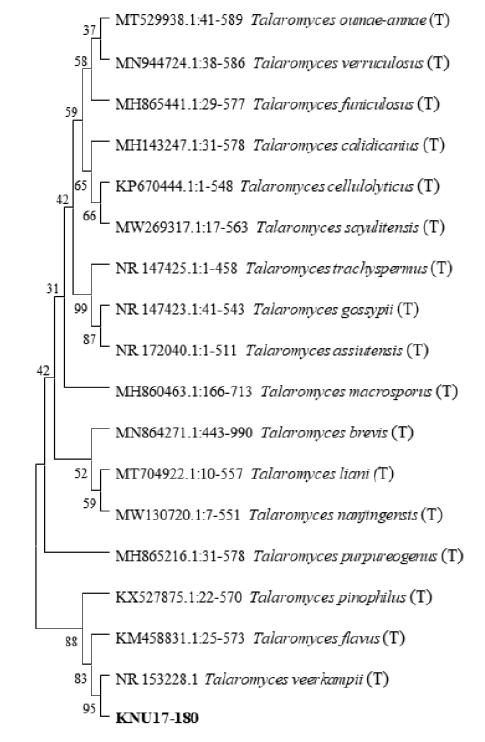
Fig. 2. Neighbor-joining phylogenetic analysis of Talaromyces veerkampii KNU17-180 obtained from field soil in Korea and analysis of its partial 18S-ITS1-5.8S-ITS2-28S rDNA region sequence. The tree was constructed using MEGAX. Sequences obtained in the study are shown in boldface. Numerical values (> 50) on branches represent the bootstrap values shown as the percentage of bootstrap replications from a 1,000-replicate analysis. ‘T’ indicates the type-strain of the isolate.
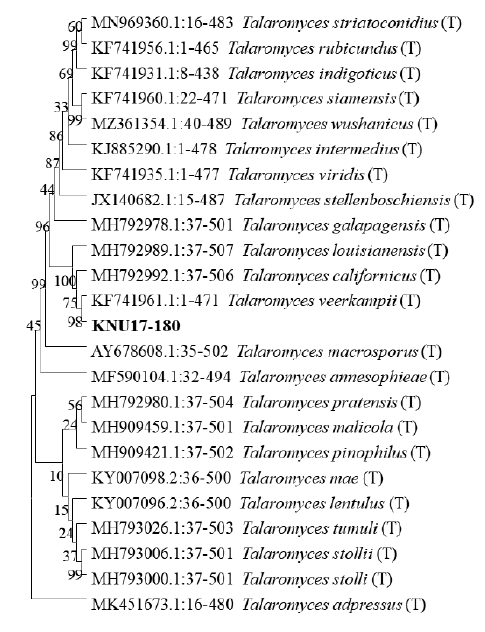
Fig. 3. Neighbor-joining phylogenetic analysis of calmodulin (CaM) gene sequences of the isolate Talaromyces veerkampii KNU17-180. The tree was constructed using MEGAX. Sequences obtained in the study are shown in boldface. Numerical values (> 50) on branches are the bootstrap values shown as percentage of bootstrap replications from a 1,000-replicate analysis. Scale bar represents the number of substitutions per site. ‘T’ indicates the type-strain of the isolate.
DISCUSSION
During a study of fungal diversity in field soils from different locations in Yangpyeong, Korea, several fungal isolates were isolated and identified using morphological and multigene molecular analyses. This study presents a newly discovered T. veerkampii fungal isolate that was identified based on morphological and multigene phylogeny. The morphological attributes of this fungal isolate were identical to those of previously reported T. veerkampii [1] with minor differences in micromorphology dimensions. The shape and structure of conidiophores, metulae, conidia, and phialides were similar to those of previously reported T. veerkampii [1]. More precisely, ampuliform-like phialides (Table 3) were exhibited by our study isolate T. veerkampii consistent with the description by Visage et al. [1]. In addition to the morphological characterization of fungal isolates, molecular techniques play a crucial role in identifying microorganisms. DNA sequencing and neighbor-joining analysis provide a highly accurate method for fungal species identification [14]. Similar to other fungi, the small and large subunit ribosomal nucleic acids and ITS sequences were conserved in Talaromyces [15]. However, ribosomal genes are too conserved to identify and differentiate the Talaromyces group of fungi [16]. Furthermore, the ITS region is currently recognized as the official DNA barcode for fungi identification [17]. In the current study, we used the ITS sequence of isolate KNU17-180 for identification. Results obtained from the phylogenetic analysis indicated that our study isolate KNU17-180 had 95% similarity with Talaromyces veerkampii (Fig. 2). Moreover, despite the identification of fungal isolates using ITS sequence variability to describe species of different sections [18], it may not be useful to differentiate members from the same section. To resolve such conflicts, the genetic marker CaM has been promoted to accurately identify fungal species [19]. The Talaromyces species can be clearly identified using the CaM sequences [15]. In the current study, we also analyzed the CaM sequence of T. veerkampii and compared it with that of the reference strains deposited in the NCBI database. Neighbor-joining analysis showed 98% similarity with previously reported T. veerkampii in the NCBI database (Fig. 3). In conclusion, the current study identified isolate KNU17-180 as T. veerkampii by morphological and molecular characterization. This newly identified Talaromyces species has not been officially reported in Korea. Therefore, we report here a new record from field soil in Korea.
ACKNOWLEDGEMENTS
This study was financed by the Ministry of Environment (MOE) of the Republic of Korea as a National Institute of Biological Resources (NIBR) grant (NIBR2014-01205) for the survey and discovery of indigenous fungal species in Korea. This study was also supported by a research grant from Kangwon National University in 2019.




