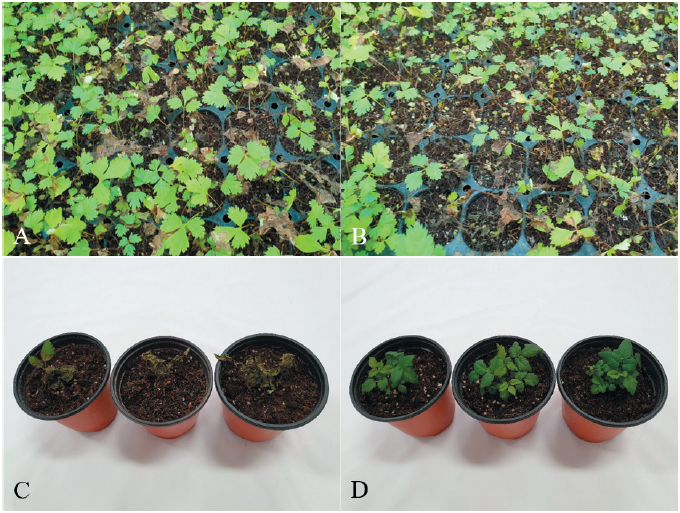
Kamchatka goatsbeard [Aruncus dioicus (Walter) Fernald] is a perennial plant that belongs to the family Rosaceae. The native range of the plant is Europe to Caucasus, East Siberia to Russian Far East, and North Central and East USA [1]. In Korea, Kamchatka goatsbeard is cultivated as a wild vegetable for human consumption. In July 2021, we surveyed diseases affecting wild vegetables grown in Hongcheon, Gangwon Province, Korea. During this survey, we observed severe seedling rot symptoms in Kamchatka goatsbeard grown in a vinyl greenhouse. The disease symptoms appeared on the leaves and petioles of the seedlings. The infected seedling parts turned brown to dark brown and rotted as the disease progressed (Figs. 1A and 1B). Five sites were observed in the vinyl greenhouse, and 100 plants at each site were investigated for the disease occurrence. The incidence of the disease in the plant seedlings was 1‒10%.
Diseased seedlings of Kamchatka goatsbeard were collected from the investigated vinyl greenhouse, and fungi were isolated from the leaves and petioles of these seedlings. The leaf and petiole lesions were cut into 3–5 mm-long pieces, surface-sterilized with 1% sodium hypochlorite solution for 1 min, and placed on 2% water agar (WA) plates. The WA plates were incubated at 25℃ for 1‒2 days, and fungal mycelia that grew from the lesion pieces were transferred onto potato dextrose agar (PDA) slants. Nine fungal isolates were obtained from the leaf and petiole lesions and used for identification. The morphological characteristics of the isolates were examined as previously described [2]. Young vegetative hyphae branched near the distal septa of the hyphal cells. Hyphae were constricted at the points of hyphal branches, and septa were formed at a short distance from the points of the hyphal branches. The isolates did not produce conidia and clamp connections on the hyphae. The morphological characteristics were identical to those of Rhizoctonia solani Kühn described previously [3, 4].
Anastomosis groups of the R. solani isolates were examined using tester isolates of R. solani (AG-1‒5), as previously described [2, 5]. All tested isolates anastomosed with the tester isolate of R. solani AG-1. The anastomosed cells of the tested isolate and the tester isolate showed killing reactions (Fig. 2A). The mycelia of the isolates cultured on PDA were light brown to grayish brown (Fig. 2B). Sclerotia were produced separately or as aggregates on the medium, and their surface was typically mycelioid or wooly. The culture type of the isolates was identified as IB of R. solani AG-1, based on comparison with previously described culture characteristics [6].
Three isolates (RS-2121, RS-2123, and RS-2125) of R. solani AG-1(IB) were tested for their pathogenicity towards Kamchatka goatsbeard seedlings through artificial inoculation, as previously described [7]. Mycelial disks of 6 mm in diameter from each isolate grown on PDA were placed on the petioles at the soil surface level of 10-month-old Kamchatka goatsbeard seedlings growing in circular plastic pots (height: 9 cm; upper diameter: 10 cm; lower diameter: 7 cm) in a vinyl greenhouse. Plastic boxes (60 cm × 43 cm × 33 cm) were used to maintain a relative humidity of 100% at room temperature (24‒26℃). The inoculated plant pots were placed in the plastic boxes for 3 days, after which they were removed from the plastic boxes and placed indoors. The inoculation test was performed in triplicate, and the results were evaluated at 5 days after inoculation. Inoculation tests showed that the tested isolates caused seedling rot symptoms in the inoculated plants (Fig. 1C), whereas no symptoms were detected in the non-inoculated plants (Fig. 1D). The induced symptoms were similar to those observed in plant seedlings from the vinyl greenhouse investigated. Re-isolation of the inoculated isolates from the lesions was confirmed.
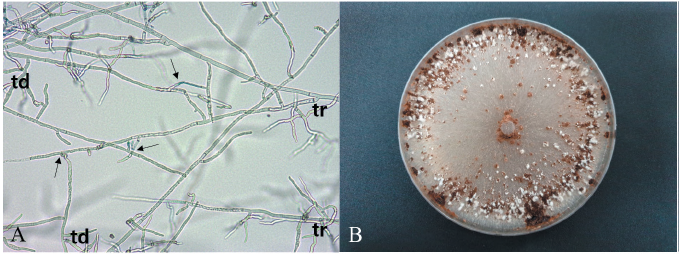
Fig. 2. Anastomosis test of Rhizoctonia solani isolate from Kamchatka goatsbeard and cultural appearance of the isolate. (A) Anastomosis reactions between the tested isolate (td) and the tester isolate (tr) of R. solani AG-1. The arrows indicate points of hyphal anastomosis. (B) Colony of R. solani AG-1(IB) isolate grown on potato dextrose agar at 25℃ for 10 days.
R. solani has very wide range of host plants, and its anastomosis groups have different genetic and pathological characteristics [4]. R. solani AG-1(IB) causes various diseases such as damping-off, leaf blight, leaf rot, stem rot, and root rot in many crops [7]. However, seedling rot of Kamchatka goatsbeard caused by the fungal anastomosis group has not been reported. We detected seedling rot of Kamchatka goatsbeard caused by R. solani AG-1 (IB).