The genus Podospora Ces. contains more than 64 species worldwide [1]. However, until recently, there was only one record of Podospora sp. isolated from the root of Calanthe discolor Lindl. (Japanese hardy orchid) as an endophytic fungus in Jeju Island, Korea. Additionally, there is no description of this species [2]. Podospora possesses unique features, such as a germ pore at the apical end of the ascospore, basal hyaline cell, and a gelatinous secondary appendage [1]. There are limited reports on asexual generation and cultural characteristics.
Various non-mycorrhizal endophytic fungi inhabit the roots of orchid plants with orchid mycorrhizal fungi [3]. Although some endophytic fungi promote seed germination of orchids [4], the research into this is currently limited. The host plant used in this study, Cypripedium guttatum Sw. (spotted lady’s slipper), is at risk of extinction due to the destruction of its native habitat and changes in the ecosystem. Therefore, the Ministry of Environment has designated it as an endangered wildlife class 1 in need of protection [5]. During the development of the artificial restoration technique of C. guttatum using a functional fungal strain, we conducted isolation and culture of endophytic fungal species and performed morphological and molecular feature analysis. Accordingly, we discovered that Podospora leporina is an unrecorded endophytic fungus in Korea.
In this study, we used samples of C. guttatum roots collected from Mt. Hambaek in Gangwon province in 2020. The collected root samples were stored in sealable zipper bags until isolation, and fungal isolation was performed within 24 hr. First, the root surface was washed with running water and then sterilized in 70% ethyl alcohol solution for 1 min and 3% sodium hypochlorite (NaClO) solution for 2 min, followed by 3 washes with distilled water. In addition, samples were treated with the antibiotics streptomycin and chloramphenicol for 10 min to prevent bacterial growth. After removing any droplets with sterilized filter paper, approximately 2-cm intervals were cut and put on water agar medium (WA; MBcell, Seoul, Korea), before being incubated in the dark at 25℃. The isolated mycelium was transferred to potato dextrose agar (PDA; MBcell) medium, and pure isolation was obtained. Morphological characteristics of pure isolation were observed through culturing in PDA medium and maltose extract agar (MEA; MBcell) medium [6]. Furthermore, microstructures were observed using potassium hydroxide and a DM2500 optical microscope (Leica Microsystems, Wetzlar, Germany). The unrecorded endophytic fungus used for observation was deposited with the Korean Collection for Type Cultures (KCTC).
Genomic DNA extraction for molecular identification was performed using a HiQ Plant DNA kit (BioD Co., Ltd., Gwangmyeong, Korea). Target DNA was amplified from the internal transcribed spacer 1 (ITS1) region to the D2 region of 28S rDNA, using ITS1 and ITS4 and LROR and LR5 primers targeting the rDNA region. The PCR conditions used in this study were implemented in previous studies [7,8]. Final PCR products were electrophoresed on 1.5% agarose gels to confirm DNA amplification, and DNA sequencing was performed by Cosmogenetech Inc. (Daejeon, Korea). The analyzed nucleotide sequence was identified using BLAST at NCBI (https://www.ncbi.nlm.nih.gov/) to determine the species with the highest similarity to the reference nucleotide sequence. A neighbor-joining tree was generated using MEGA 10.0.5 based on the Kimura-2 parameter distance model with the 1,000-times bootstrap method [9].
Podospora leporina (Cain) Cain, Can. J. Bot. 40: 460 (1962) [MB#337412]
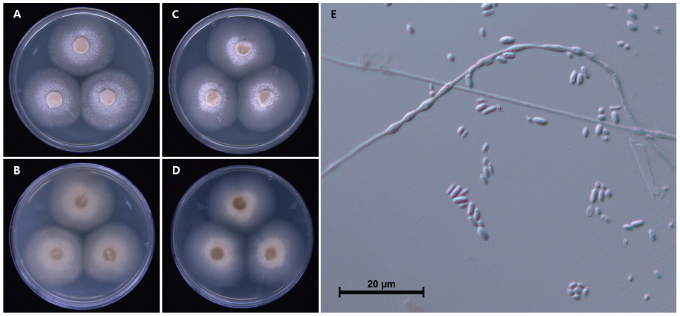
The colony diameter after 14 days was 85.3-93.5 mm on MEA and 81.5-96.6 mm on PDA. The mycelium was relatively loose. The surface color was goldish gray (Munsell color notation: 2.5GY 7/2) to dark phthalo bluish gray (Munsell color notation: 5PB 4/2) in MEA and light Persian bluish gray (Munsell color notation: 10PB 7/4) to phthalo bluish gray (Munsell color notation: 7.5PB 5/4) in PDA. Moreover, the texture was similar to loose cotton in the center and smooth in the margin; the colony was circular with no exudate. The reverse was close to an orchid gray (Munsell color notation: 5P 6/2) in MEA and PDA [10]. Conidia measured about 2.5–4.4×1.0–2.3 μm (n=20), were blunt rods with round ends, hyaline, dyed well with lactophenol cotton blue, and had no septa inside (Fig. 1; Table 1).
Table 1. Morphological characteristics of Podospora leporina CJO34015 isolated from the root of Cypripedium guttatum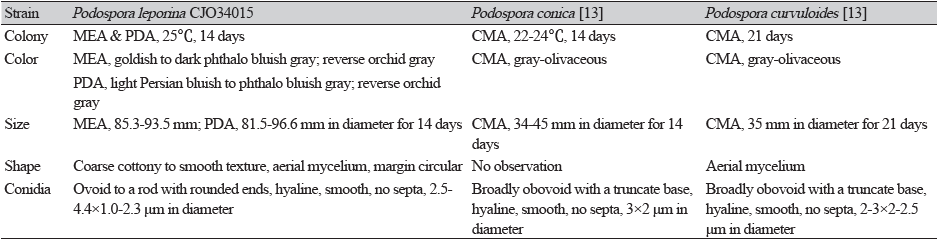
|
|
MEA: malt extract agar; PDA: potato dextrose agar; CMA: corn meal agar |
Specimen examined: Mt. Hambaek, Gangwon-do, Korea, 37°8'57.78''N, 128°54'10.80''E, 2020.10.6., isolated from the root of C. guttatum, strain CJO34015, KCTC no. 56904, GenBank nos. OP445245 (ITS) and OP445246 (LSU).
Although more than 64 species in the genus Podospora are distributed worldwide, there is no description of this genus or any single species in Korea. In the case of P. leporina, Cain initially reported it as Sphaeria leporina in 1934 and then re-organized it into the genus Podospora in 1962 [11,12]. To date, Podospora spp. has mainly been found and reported in the excrement of various animals. However, in our study, we found this fungus in the root of the spotted lady’s slipper orchid. In a previous study, Podospora sp. was similarly isolated from the root of the Japanese hardy orchid, so further research is required to understand these affinities. In the BLAST results, ITS sequence of P. leporina showed 98.2% similarity with P. leporina MH859324 and LSU 98.6% similarity with P. leporina MH871063. Phylogenetic analysis from ITS and LSU sequences using neighbor-joining method placed the P. leporina in a separate clade with the reference sequences (Fig. 2). Additionally, many of the previous studies on the morphology of Podospora spp. were based on the ascus and its spores, which were directly isolated from animal feces. Our morphological observations in the medium environment of the laboratory are insufficient, and thus, further taxonomic research is warranted.
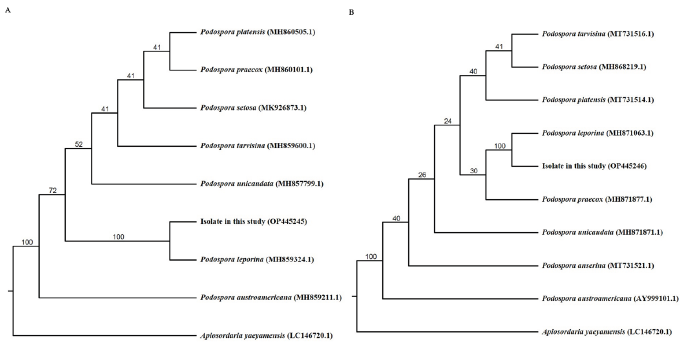
Fig. 2. Phylogenic tree of Podospora leporina CJO34015 isolated from Cypripedium guttatum root. A: The internal transcribed spacer (ITS) region, including 5.8S rDNA, and (B) 28S rDNA, including the D1 and D2 regions, were used for sequence analysis to confirm the topological appropriation of the fungal isolates. Bootstrap values are shown at the branches (1,000 replicates).