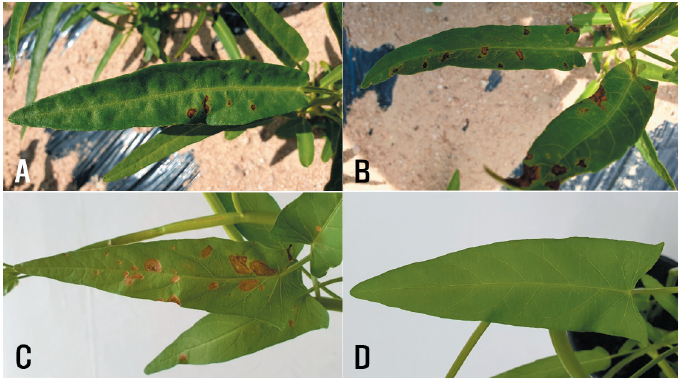
Water spinach (Ipomoea aquatica Forssk.) belongs to the family Convolvulaceae and is native to Africa and Southeast Asia [1]. This plant is used as a food and medicinal material [2]. It is semi-aquatic and is widely cultivated as a vegetable in many counties of Eastern and Southeastern Asia. It was also introduced to Korea many years ago and has since been grown as a vegetable ingredient. Leaf spot symptoms were observed in water spinach plants growing in fields in Ansan and Hongseong, Korea, during disease surveys in 2019 and 2020. The symptoms appeared as brown to dark brown circular or irregular spots on the leaves of the plants (Figs. 1A and B). Three sites were observed in the field, and 100 leaves of the plants at each site were investigated for disease incidence. The disease incidence on the plant leaves in the fields investigated at the two locations ranged from 1% to 20% (Table 1).
Diseased water spinach leaves were collected from the investigated fields. Fungal isolates were obtained from pieces of lesions in the diseased leaves using the methods as described in a previous report [3]. Most of the isolates were identified as Phoma sp. based on a comparison of their morphological characteristics with those described by Boerema et al. [4]. Five single-spore isolates were obtained from the original isolates and used for further identification and pathogenicity tests.
The single-spore isolates were cultured on malt extract agar (MEA), oatmeal agar (OA), and potato dextrose agar (PDA) at 22℃ for 14 days to investigate their cultural and morphological characteristics using the methods described in the previous reports [4, 5]. The average diameters of 7-day-old colonies of the isolates grown on MEA, OA, and PDA in the dark were 5.7 cm, 6.9 cm, and 4.9 cm, respectively. NaOH spot tests [4] of the isolates on 7-day-old cultures on MEA showed negative reactions. The colonies on MEA showed an undulate margin, white sparse aerial mycelia, and a brown center with aggregate pycnidial formation (Fig. 2A). The colonies on OA showed a colorless margin and chestnut to brown aerial mycelia with repeated white rings (Fig. 2B). The colonies on PDA showed traits similar to those on MEA but with less pycnidial formation (Fig. 2C). The morphological characteristics of each isolate were investigated using 30 pycnida and 30 conidia from 2-week-old cultures on OA. The pycnidia varied in size and shape; they were dark brown to black in color, mostly globose to subglobose, and solitary or confluent (Fig. 2D), and measured 98.8‒407.0 μm in diameter. The conidia were ellipsoidal to oblong with mostly bipolar or multiple guttules, mainly aseptate (Fig. 2E), and measured 2.5‒8.9 × 1.0‒3.9 μm (av. 4.4 × 2.1 μm). All the isolates were identified as Phoma multirostrata (P.N. Mathur et al.) Dorenb. & Boerema based on the cultural and morphological characteristics described previously [6]. P. multirostrata was reclassified as Ectophoma multirostrata (P.N. Mathur, S.K. Menon & Thirum.) Valenz.-Lopez, Cano, Crous, Guarro & Stchigel based on a phylogenetic study [7].
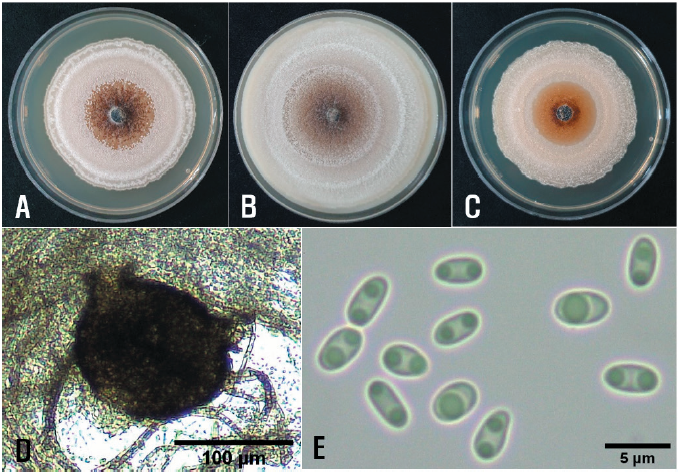
Fig. 2. Cultural and morphological features of Ectophoma multirostrata isolate from water spinach. Colonies of the isolate grown on malt extract agar (A), oatmeal agar (B), and potato dextrose agar (C) at 22℃ for 14 days. A pycnidium of the isolate produced on oatmeal agar (D) and conidia produced in the pycnidium (E).
To verify the identiy of the isolates based on their cultural and morphological characteristics, partial large subunit nrDNA (LSU), internal transcribed spacer regions 1 & 2 and intervening 5.8S nrDNA (ITS), and partial gene regions of β-tubulin (TUB2) of the isolates were investigated with LR0R [8] and LR7 [9], V9G [10] and ITS4 [11], and TUB2Fd and TUB4Rd [12], respectively. Genomic DNA of the isolates was extracted using the protocol described by Blount et al. [13] with slight modification. The conditions for PCR amplification of all the genes were set according to a previous study [14]. Genomic DNA was sequenced using primer sets produced by Bionics (Seoul, Korea). To construct a phylogenetic tree of the concatenated sequences with the three target genes, the maximum likelihood (ML) method with 1,000 bootstrap replicates was implemented using randomized axelerated maximum likelihood (RAxML) GUI version 2.0 [15], based on a general time-reversible substitution model with inverse gamma-distributed rate variation (GTR+G+I). The final concatenated alignments included 12 ingroup taxa with a total of 1,665 characters including gaps (908 for LSU, 429 for ITS, and 328 for TUB2).
Coniothyrium palmarum (CBS 400.71) was selected as the outgroup taxon. The phylogenetic tree based on the concatenated sequence data showed that all the isolates were clustered in a group with E. multirostrata CBS 274.60 (Fig. 3). The nucleotide sequences of the LSU, ITS, and TUB2 genes obtained from the five isolates were deposited in GenBank with accession numbers OM392039–OM392043, OM392044–OM392048, and OM417603–OM417607, respectively.
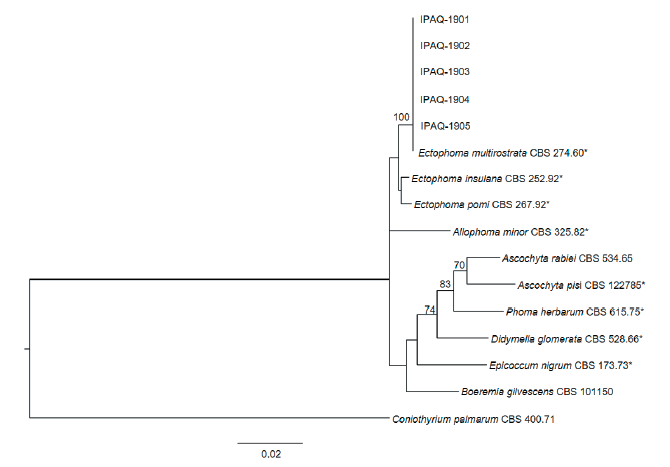
Fig. 3. Phylogenetic tree based on the concatenated sequences of partial large subunit nrDNA, internal transcribed spacer regions 1 & 2 and 5.8S nrDNA, and β-tubulin of five isolates (IPAQ-1901–IPAQ- 1905) from water spinach and reference species. The reference sequence data were obtained from the NCBI GenBank database. The tree was generated using the maximum likelihood method based on a general time-reversible substitution model with inverse gamma-distributed rate variation (GTR+G+I) in RAxML GUI version 2.0. The bootstrap support values are given at the nodes. The bar represents the number of nucleotide substitutions per site. Representative strains are marked by an asterisk (*).
Two isolates of E. multirostrata were used to confirm their pathogenicity in water spinach plants via artificial inoculation. A conidial suspension (1–2 × 106 conidia/mL) of each isolate was prepared from 2-week-old OA cultures. The conidial suspension was sprayed onto 33-day-old water spinach plants (30 mL onto each plant) grown in plastic pots (height: 13.5 cm; upper diameter: 15 cm; lower diameter: 9 cm) in a vinyl greenhouse. Control plants were treated with 30 mL of sterile distilled water. Inoculated plants were placed in plastic boxes (71.0 × 53.5 × 40.5 cm) under 100% relative humidity at room temperature (24–26℃) for 5 days. The inoculated plants were then moved outside the boxes and placed in a vinyl greenhouse. Ten days after inoculation, the pathogenicity of the isolates was determined based on the degree of leaf spot symptoms. The pathogenicity test was conducted in triplicate. All the tested isolates caused leaf spot symptoms in the inoculated plants (Fig. 1C); however, no symptoms were observed in the control plants (Fig. 1D). The symptoms of the inoculated plants were similar to those observed in the investigated fields. Re-isolation of the inoculated isolates from the lesions was confirmed.
In this study, the fungal isolates causing leaf spot in water spinach plants in Korea were identified as E. multirostrata. Cercospora ipomoeae G. Winter, Phyllosticta ipomoeae Ellis & Kellerm., and Pseudomonas syringae pv. syringae van Hall have been reported to cause leaf spot in water spinach [16]. However, E. multirostrata has never been reported as a leaf spot pathogen in water spinach. To our knowledge, this is the first report of E. multirostrata causing leaf spot in water spinach.