INTRODUCTION
Botryosphaeriaceae is a family of fungi that belong to the order Botryosphaeriales. A total of 58 genera have been described in the family Botryosphaeriaceae and 240 species have been identified in the genus Botryosphaeria [1]. Botryosphaeria is a wide genus in the family Botryosphaeriaceae and was first reported by Cesati and de Notaris [2]. Botryosphaeria has been reported to be a plant pathogenic, endophytic, and saprobic fungus [3-5], and various species of this genus are pathogens of different crops and important trees worldwide [6]. Several species have been shown to act as opportunistic pathogens with little symptoms or as endophytes without symptoms under stressful circumstances [6]. Botryosphaeria dothidea from the genus Botryosphaeria, is known for its widespread distribution to a large number of hosts [4,6,7]. Moreover, B. dothidea was reported to cause disease in several crops such as apple and plumcot trees in Korea [8,9]. However, Botryosphaeria comprises a variety of morphologically diverse pathogens, endophytes, and saprobes that thrive primarily on woody hosts. Much of the interest in these fungi has centered on the systematics of species and genera due to their frequent association with plant diseases [4].
In this study, we report Botryosphaeria kuwatsukai which was obtained from apple tree barks during exploration of fungi in Korea. Cultural and morphological characteristics and molecular phylogeny of B. kuwatsukai strains were described. This species is a new record in Korea.
MATERIAL AND METHODS
Sample collection and fungal isolation
Declined apple tree samples with darkening on the trunk, dark-brown spots, and embedded pycnidia on the bark (Figs. 1A-C) were collected during screening for fungal species at apple orchards in Muju-gun and Mungyeong-si in Gyeongsangbuk-do and Yeocheon-si in Jeollanam-do, Korea. To isolate the causal agent, the pycnidial structures were taken from the abnormal bark, transferred onto potato dextrose agar (PDA; Difco, Detroit, MI, USA), and incubated at 25℃. Then single mycelium or even conidiophores were isolated and incubated on PDA at 25℃ [10]. As a result, three strains designated as KNUF-23-MG32, KNUF-23-YC8, and KNUF-23-MJ82 were isolated from infected apple trees and selected for further morphological and molecular evaluation. For further study, the fungal strains were maintained in 20% glycerol at -80℃.
Cultural and morphological characterization
Cultural characteristics and morphological observations of the fungal strains KNUF-23-MG32, KNUF23-YC8, and KNUF-23-MJ82 were evaluated in PDA media and 2% malt extract agar (MEA; Difco, Detroit, MI, USA) with incubation for 4 days at 25℃ [11]. Conidiomata were observed in pine needle agar (2% water agar with sterilized pine needle) for 14 days. Fungal growth was quantified, and colony details, including color, shape, and size were noted. A light microscope (BX-50, Olympus, Tokyo, Japan) was used to observe the morphological properties.
Genomic DNA extraction, PCR amplification, and sequencing
Total genomic DNA was isolated from fungal mycelia of strains KNUF-23-MG32, KNUF-23-YC8, and KNUF-23-MJ82 grown on a PDA plate using the HiGeneTM Genomic DNA Prep Kit (BIOFACT, Daejeon, Korea) according to the manufacturer's protocol. The ITS1F/ITS4 primer pair was used to amplify the internal transcribed spacer (ITS) regions [12,13]. A fragment of β-tubulin (TUB2) was amplified using the Bt2a/Bt2b primer [14], while EF1-688F/EF1-1251R was used to amplify the translation elongation factor 1-alpha (TEF1α) [15]. To assess the quality of PCR products, electrophoresis using 1.2% agarose gel stained with ethidium bromide was performed. The PCR products were purified using ExoSAP-IT (Thermo Fisher Scientific, Waltham, MA, USA) and sequenced by SolGent (Daejeon, Korea).
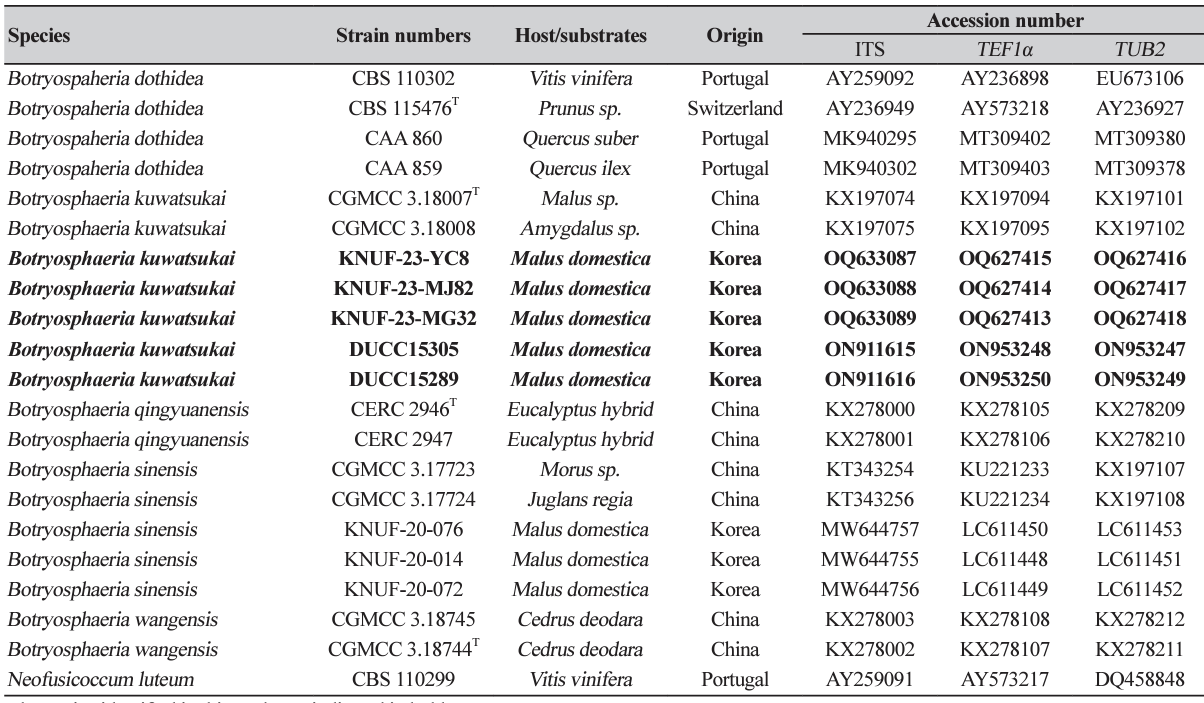
Fig. 1
Natural apple decline symptoms of Botryosphaeria kuwatsukai. A declined apple tree of an orchard (A); black dots on a diseased trunk (B, C); front and reverse colony morphologies grown on potato dextrose agar (D) and malt extract agar (E), respectively, at 5 days after incubation (DAI) and 25℃. Pycnidia on pine needle agar (PNA) medium (F); conidiophores and conidiogenous cells (G); Conidia (H). Pathogenicity test on Fuji apple fruits using the strain KNUF-23-MG32 (I-N). Control fruit inoculated with mycelial potato dextrose agar (PDA) plugs for 7 days at 25℃ (I); rotted and brown colored fruit (J); magnified photo of J (K); control fruit inoculated using sterile needles (L); necronized and brown colored fruit (M); magnified photo of M (N); Scale bars: 20 µm.
Pathogenicity test
Healthy apple fruits (Fuji apple) were used to perform pathogenicity test of the strain KNUF-23-MG32. Briefly, the fruits at the same developmental stages were selected and then surface sterilized with 70% ethanol. Mycelial plugs (4-5 mm) from a five-day-old colony and inoculum (1×105 conidia/mL) were prepared to inoculate into fruits. PDA plugs and paper discs dipped in double distilled water were used as control. Fresh wound sites were created on the fruits using sterile needles and then PDA plugs and paper discs were attached. The inoculated spots were wrapped with parafilm to maintain moisture and incubated at 25℃ in a growth chamber for 3-5 days [10]. The pathogenicity experiment was conducted with three replicates on fruits. The formation of fruit rot caused by fungi was visually observed and photographs were taken.
Molecular phylogenetic analysis
The phylogenetic analysis of the strains KNUF-23-MG32, KNUF-23-YC8, and KNUF-23-MJ82 strains was performed with the determined nucleotide sequences of the internal transcribed spacer (ITS) regions, translation elongation factor 1-alpha (TEF1α), and partial β-tubulin (TUB2). Reference sequences of Botryosphaeria spp. were retrieved from the National Center for Biotechnology Information (Table 1). The ambiguous regions were deleted manually from the alignments, and Kimura's two-parameter model was used to compute the evolutionary distance matrices needed for the neighbor-joining (NJ) algorithm [16]. The phylogenetic tree was constructed with the NJ method to infer the tree topology with 1,000 bootstrap replicates in MEGA7.0 software [17].
RESULTS AND DISCUSSION
Botryosphaeria kuwatsukai (Hara) G.Y. Sun & E. Tanaka, Fungal Diversity 71: 225 (2015) (Fig. 1)
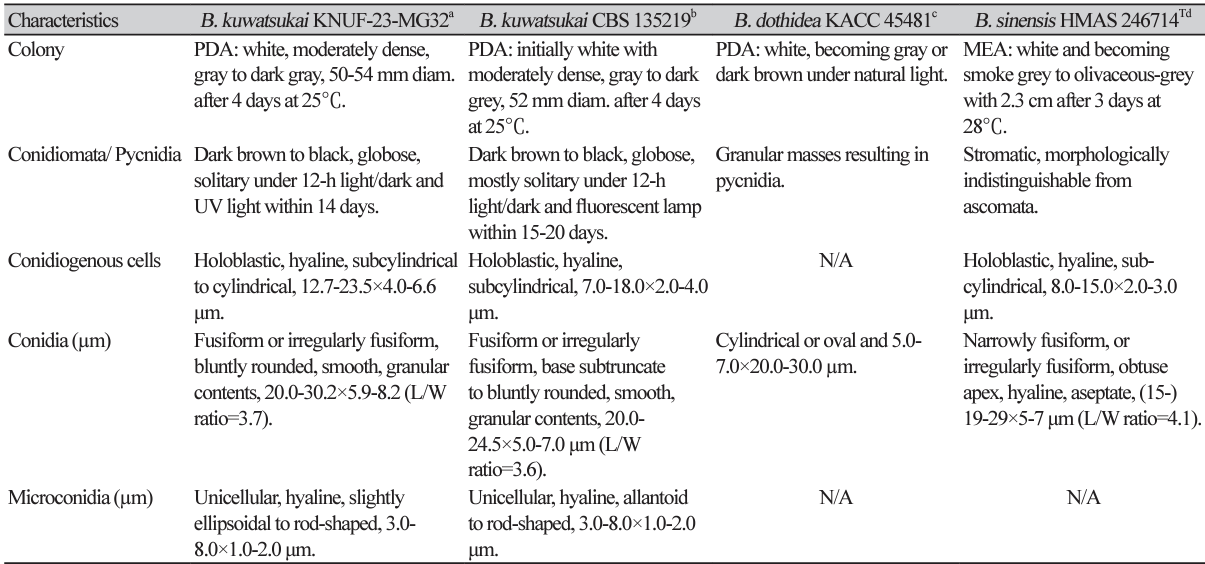
Analyses of the three strains, KNUF-23-MG32, KNUF-23-YC8, and KNUF-23-MJ82, revealed similar cultural and morphological characteristics along with their molecular phylogeny. Therefore, the cultural and morphological characteristics of the KNUF-23-MG32 are described as a representative strain in this study and identified as Botryosphaeria kuwatsukai.
Morphology of the strain KNUF-23-MG32
The colonies achieved a diameter of 50-54 mm on PDA at 4 days after incubation (DAI) at 25℃, which were primarily white to moderately dense, with abundant aerial mycelia, colonies gradually turning gray to dark gray; the reverse side of the colonies initially white before turning dark green to olive-green from the center. The colony color spreads to the edge and turns dark to black (Fig. 1D). The colonies on MEA had almost similar features, but with a slightly lower growth rate attaining 48-51 mm at 4 DAI (Fig. 1E). Conidiomata was absent in culture on PDA and MEA even in interactive treatment with black and UV light at 14 DAI, colonies were superficial, dark brown to black, globose, mostly solitary, and covered by the mycelium on pine needle agar at 7 DAI (Fig. 1F). A globose, dark-brown to black microconidiomata was observed, while microconidiophores were rare. Conidiogenous cells were holoblastic, hyaline, subcylindrical, 12.7-23.5 ×4-6.6 μm (n=10) (Fig. 1G). Microconidia were unicellular, hyaline, slightly ellipsoidal to rod-shaped, 2.0-8.8 ×1.5-3.7 (5.4×2.8, n=30) μm. Conidia were abundantly produced, and were hyaline, narrowly or irregularly fusiform, bluntly rounded, smooth, with granular contents inside, widest from middle to upper, 20.0-30.2×5.98.2 μm (26.3×7.2; n=50, length/width [L/W] ratio=3.7) (Fig. 1H). Sexual state was not observed in culture. The morphological and cultural characteristics of strain KNUF-23-MG32 closely resembled features of the previously identified Botryosphaeria kuwatsukai (Table 2).
Pathogenicity test
All treated apple cv. Fuji fruits showed rot or necrosis symptoms with dark-brown discolorations, brown spots, and darkening fruits after 5-7 days of inoculation (Figs. 1J, K, M, N). Whereas no symptoms were observed in the control fruits inoculated using the mycelial plug method (Fig. 1I) and using a sterile needle (Fig. 1L). The pathogen was re-isolated and we confirmed Koch’s postulates.
Molecular phylogeny of the strain KNUF-23-MG32
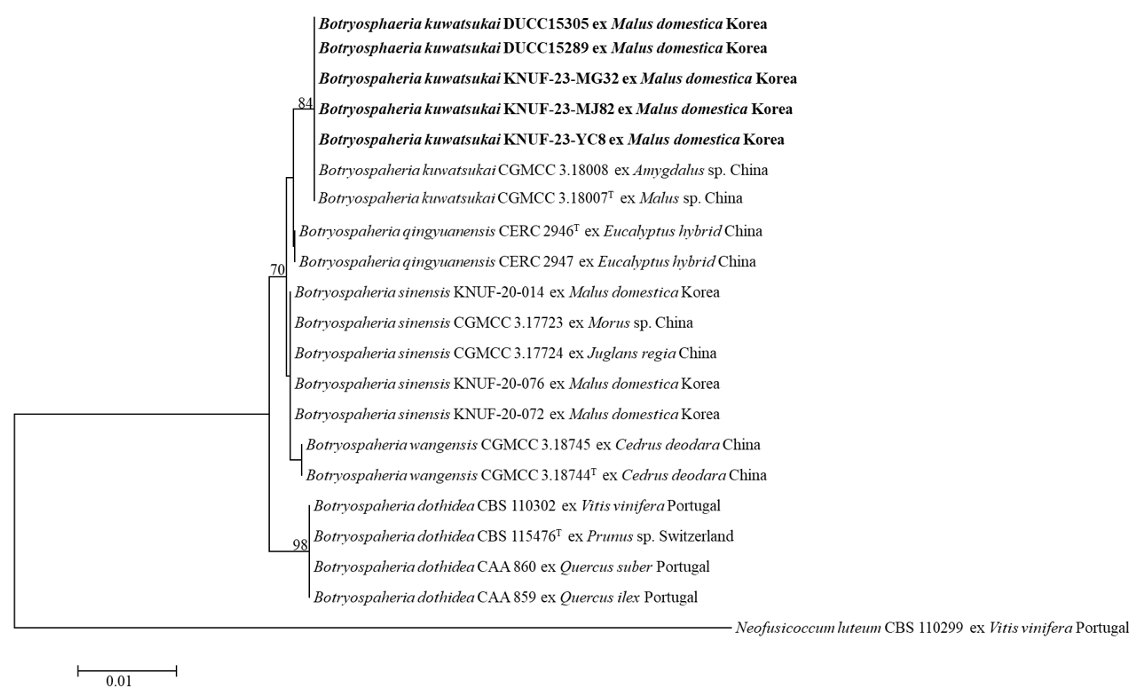
After sequencing, nucleotide sequences of ITS (931, 783, 908 bp), TEF1α (519, 458, 521 bp), and TUB2 (438, 439, 437 bp) were determined from three strains (KNUF-23-YC8, KNUF-23-MJ82, and KNUF-23MG32), respectively. These determined sequences of the three strains were deposited in GenBank database with accession numbers (Table 1). The BLAST results of the ITS regions from the three strains revealed 99.8-100% similarity with Botryosphaeria kuwatsukai (KUMCC 20-0106), and 99-100% similarity with B. dothidea (MGEF23 and KNUF-22-MG31), and B. fusispora (MFLUCC 10-0098 and GBLZ16BO-020), respectively. The TEF1α sequences of the three strains displayed 99.6-100% similarities with B. kuwatsukai (DUCC15305, DUCC15289, and CFCC 82350) and 99-100% similarity with B. dothidea (PD313, ED2311, SXG1331, and MPWL93), and B. fusispora (CPC 29629 and CBS 118831). In the case of TUB2, the three strains showed 100% similarities with B. kuwatsukai (DUCC15305, DUCC15289, CFCC 82350, and CDZM742), and 99.8-100% similarities with B. dothidea (JZB310177, JZB310176, and GZHGS-2017-010). The phylogenetic tree constructed based on the combined ITS regions, TEF1α, and TUB2 showed that the three strains (KNUF-23-YC8, KNUF-23-MJ82, and KNUF-23-MG32) clustered with the strains of B. kuwatsukai (Fig. 2). Consequently, the three isolated fungal strains in this study from the apple orchards were identified as B. kuwatsukai. DUCC15305 and DUCC15289 strains of B. kuwatsukai which were phylogenetically conformed with KNUF-23-YC8, KNUF-23-MJ82, and KNUF-23-MG32 strains (Fig. 2) were from the cankered apple tree twigs in apple orchards in Korea. Their colony characters and multi-locus gene sequences similar to the three strains of B. kuwatsukai from this study. But their description has not been published and thus included in this study. These DUCC15305 and DUCC15289 strains were deposited at National Institute of Biological Resources with the registered number NIBRFGC000509985. This is a new record of Botryosphaeria fungal pathogen occurring in apple orchards in Korea.
Fig. 2
Neighbor-joining phylogenetic tree based on internal transcribed spacer (ITS), translation elongation factor 1-alpha (TEF1α) gene, and β-tubulin (TUB2) gene sequences, showing the relationship of Botryosphaeria kuwatsukai and the closest Botryosphaeria species. Neofusicoccum luteum (CBS 110299) was used as an outgroup. The numbers above the branches indicate bootstrap values (>70%) obtained from 1,000 replicates. The strain isolated in this study is indicated in bold. Bar=0.01 substitutions per nucleotide position.
Botryosphaeria species are globally distributed pathogenic plant saprobes and endophytes that predominantly affect woody hosts [7,20], with frequent occurrence in plants under environmental stresses, such as drought, frost, physical injury, and biotic stresses [6,21]. B. sinensis has previously been reported in the twigs of Populus sp., Morus alba, and Juglans regia from China [19], on Paulownia tomentosa and Prunus sp. from Japan [22], and on Mangifera indica from Australia [23]. Whereas B. dothidea is known to be associated with the Chinese hickory trunk canker [24] and B. laricina causes shoot blight on Larix sp. [25]. B. sinensis associated with the apple decline has frequently been detected on Fuji apple trees which is a popular cultivar in Korean apple orchards [10]. Moreover, B. dothidea is responsible for stem rot disease in Plumcot trees, while B. parva has been associated with stem blight on Rubus crataegifolius in Korea [9,26]. B. kuwatsukai was previously detected on an apple (Malus domestica) tree branch in China [11]. Also, apple ring rot disease was first reported in Japan in 1907 and later the pathogen was described as Macrophoma kuwatsukai (Currently Botryosphaeria kuwatsukai) [27]. In this study, B. kuwatsukai was described as apple ring rot since this name was commonly reported [28,29]. Since it was isolated from apple tree twigs and tree barks in Korea, further study needs to be done about its impact in domestic apple production. The genus Botryosphaeria includes diverse species [1], some of which inflicts catastrophic damage to various economically important agricultural crops. Our repost of unrecorded B. kuwatsukai in this study will improve our knowledge of Botryosphaeria distribution and help to understand its relationship with other important Botryosphaeria species on apple such as B. dothidea and B. sinensis and with other hosts in Korea.