INTRODUCTION
The genus Tuber (Pezizales, Ascomycota) comprises over 180 species worldwide [1,2]. These fungi form ectomycorrhizal (ECM) relationships with certain host plants, such as oak or hazel [3], leading to the production of subterranean fruiting bodies [4]. Although most ECM fungi form symbiotic relationships with a number of different host plant species [5,6], Tuber spp. exhibit high selectivity for specific hosts [1]. For example, Tuber gibbosum and T. oregonense establish unique symbiotic relationships with Pseudotsuga menziesii [2].
In Greece, the optimal host species for truffle cultivation include Quercus coccifera, Q. ilex, and Q. pubescens [7]. Indeed, careful selection of the host species is crucial for successful truffle cultivation. Moreover, each Tuber species exhibits unique morphological or anatomical characteristics when forming ECM associations, underlining the importance of understanding these characteristics for their cultivation [8,9].
In September of 2020, fruiting bodies were collected from the rhizosphere of Q. aliena in Gyeongju, Korea. Based on their morphological characteristics and molecular analysis, we identified T. koreanum as a new Tuber species [10]. Since then, T. koreanum has also been found in the rhizosphere of Q. aliena in both Mungyeong and Uljin.
In the present study, we sought to characterize the ECM associations formed by T. koreanum when inoculated into two different oak tree species commonly found in Korea, Q. acutissima and Q. dentata. Quercus acutissima was chosen as it is representative of the oak tree species frequently found in Korea, whereas Q. dentata was selected owing to its role as a host plant for other Tuber spp. indigenous to Korea [11,12].
MATERIAL AND METHODS
Seeds of Q. acutissima were collected from Danyang-gun, Korea, whereas those of Q. dentata were collected from Yeongju-si, Korea. The collected seeds were then de-shelled and their surfaces sterilized in 10% sodium hypochlorite (NaOCl) for 30 min. Following sterilization, each seed was placed in a plastic pot (SC10, Stuewe and Sons., Inc, Oregon, USA) containing an autoclaved soil mixture (1:1 ratio of vermiculite to perlite, sterilized for 1 h at 121℃) and incubated in a greenhouse at 25℃. Subsequently, seeds were maintained in a growth chamber under controlled conditions (8-h photoperiod per day, 55±5% relative humidity, and 24±1℃ temperature) for 8 months.
The fruiting bodies of T. koreanum were collected from the rhizosphere of Q. aliena in Gyeongju-si, Korea [10]. The T. koreanum ascocarps were rinsed with tap water, sterilized with 70% EtOH, and ground with sterile water using a blender (SGMF-650, Hanil, Seoul, Korea), resulting in a spore suspension. Each previously prepared seedling was inoculated with 1 mL of T. koreanum spore suspension (1. 0×105 spores/mL). At the time of inoculation, the spore suspension was applied near the root. In total, ten inoculated seedlings were produced, comprised of two different species combinations (Q. acutissima×T. koreanum and Q. dentata×T. koreanum) with five trees each. The growth area of the inoculated seedlings was uniformly maintained, the soil was watered to retain moisture, and the pH was maintained at 8 by applying with slaked lime.
Approximately 8 months of inoculation, the ECM-associated root tips were classified based on their morphological characteristics, and representative root tips from each group were selected for molecular identification. To determine the successful formation of T. koreanum ECM in each inoculated seedling, DNA was extracted from the root tips using a DNeasy Plant Mini kit (Qiagen GmbH, Hilden, Germany). Polymerase chatin reaction (PCR) was conducted using ITS1F/ITS4 primers [13] and DNA sequencing was performed (SolGent Co., Ltd., Daejeon, Korea). Using NCBI BLAST (https://www.ncbi.nlm.nih.gov/), species showing the highest agreement were identified for the nucleotide sequence analysis.
Colonization rates (CRs) were assessed eight months after inoculation. From each seedling, the soil near the root was removed and washed with distilled water, and three root sections (2-3 cm each) were randomly sampled. The colonization rate was calculated by counting the total number of T. koreanum, ECM root parts, non-ECM root parts, and contaminated root parts from the extracted roots. A total of 100 root sections from each plant species were examined, and the results are expressed as the percentage of tips infected with T. koreanum. Statistical analysis were performed using SPSS (version 26, IBM Corp., NY, USA). Statistical differences between the measured data of each host plant were compared using an independent student t-test.
Eight months after inoculation, the mycorrhizal characteristics of T. koreanum formed on the roots of each seedling were observed. The morphological characteristics of the ECM were examined and recorded using a stereomicroscope (Olympus SZX7, Olympus, Tokyo, Japan). Sections were prepared using a frozen microtome (LEICACM1850, Leica, Heidelberg, Germany), and ECM anatomical properties were observed and recorded using an optical microscope (Axio Imager A1, Carl ZEISS, Oberkochen, Germany).
RESULTS AND DISCUSSION
Approximately 8 months after inoculation with the T. koreanum spore suspension, mycorrhizal formation was observed. For both host plant species, T. koreanum successfully colonized all five seedlings. The BLAST results showed that the internal transcribed spacer (ITS) sequences of all observed ECM associations were closely related to those of the ascoma (GenBank accession no. AB553502), with more than 98% similarity, confirming that the DNA sequence of the ECM belonged to the same phylogenetic group as the ITS sequence of the ascoma inoculated.
Upon measuring CRs in both host plant species, the mean CR for the Q. acutissima×T. koreanum combination was 0.46±0.09, whereas that for the Q. dentata×T. koreanum combination was 0.31± 0.03. While the average CR appeared to be higher in Q. acutissima than that in Q. dentata, the difference was not statistically significant.
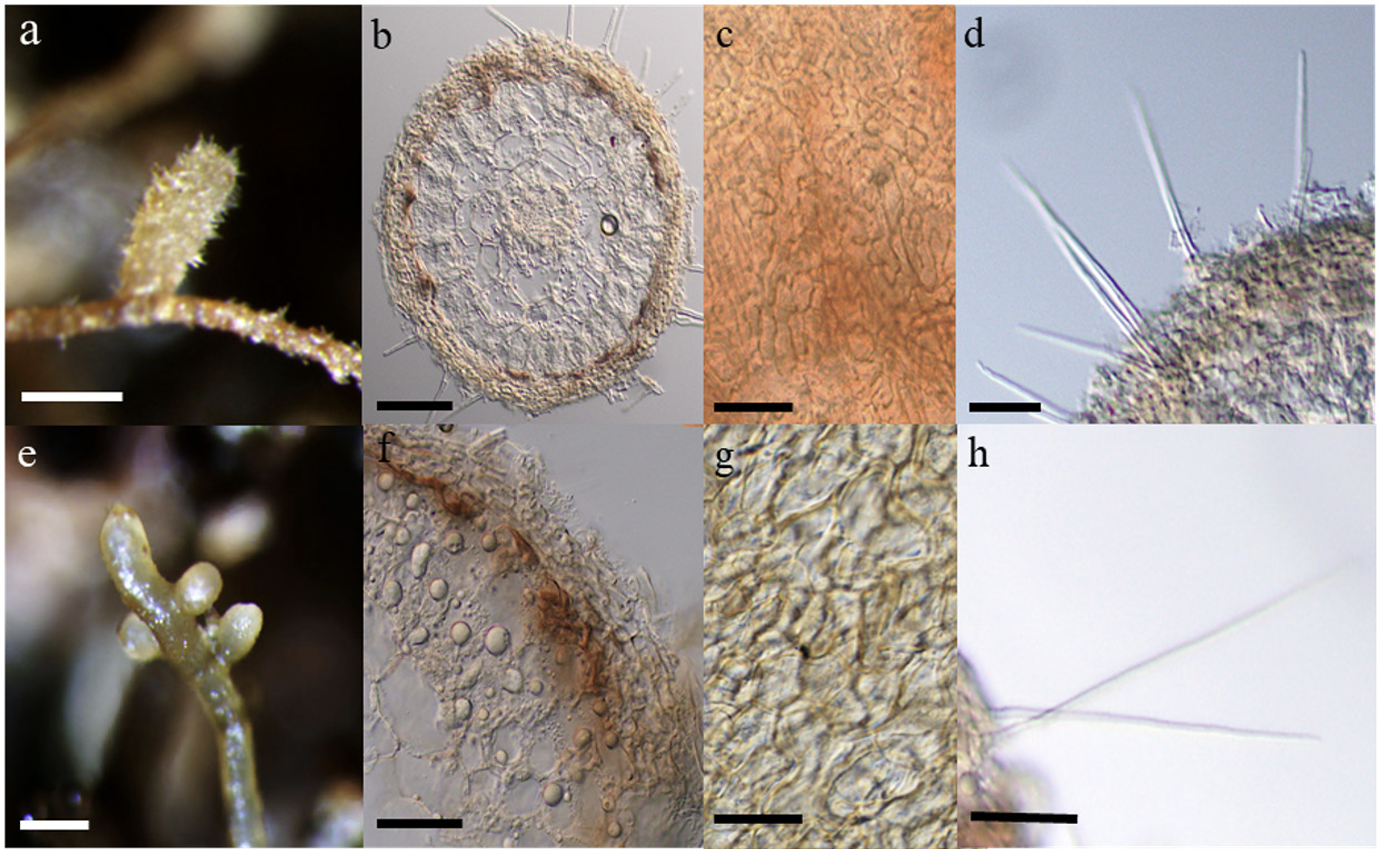
Eight months after inoculation, the ECM formed in Q. acutissima and Q. dentata were examined. The morphological and anatomical characteristics of the ECM formed in each host plant are presented in Fig. 1, and the corresponding measured values are listed in Table 1. In both host plant species, the unramified ends were straight, club shaped, or cylindrical with rounded ends. The mantle is characterized by an irregular pattern of interlocking hyphae, which are simple, bristle-like, awl-shaped, and non-septate.
Different mycorrhizal properties were observed in the ECM associations formed with the two host plant species. Both monopodial pinnate and pyramidal patterns were observed in that of Q. acutissima, whereas only the monopodial pinnate pattern was observed in that of Q. dentata. The ECM of Q. acutissima was whitish-light brown, brown, or reddish-brown in color, whereas the ECM of Q. dentata was either pale yellow or ochre. Furthermore, the ECM system was significantly longer in Q. dentata than in Q. acutissima. Additionally, the length and diameter of the unramified ends and cystidia were significantly greater in Q. dentata than in Q. acutissima. In contrast, mantle thickness was significantly larger in Q. acutissima than in Q. dentata (Table 1).
Most Tuber spp. exhibit host selectivity at the species level [14]. In fact, some species have been found to have higher affinity with certain species of oak tree than others [7,15]. When T. koreanum was inoculated into indigenous host plant species and the CRs were compared, a higher CR was observed in Q. acutissima than in Q. dentata. These findings suggest that T. koreanum may exhibit a preference for this specific host plant species. Selecting suitable indigenous host plants is important for the successful mycorrhization of Tuber spp. [1]. Although this study compared the CRs of two species, further studies comparing the CRs of a larger range of host plant species are warranted. This study will facilitate more effective cultivation of T. koreanum by helping to identify optimal host plant species.
Fig. 1
Macro-morphological and anatomical characters of Tuber koreanum mycorrhizae with Quercus acutissima (a-d) and Q. dentata (eh). The shape of mycorrhizal root tips (a & e); Cross section of mycorrhizal root tips (b & f); Outer mantle surface structure (c & g); Separate hyphae emanating from outer mantle layer (d & h) (scale bars: a, e=1 mm; b, c, d, h=50 μm; f, g=20 μm).
In the present study, we investigated the formation and characteristics of ECM produced by T. koreanum when associated with either Q. acutissima or Q. dentata. For the first time, this study describes ECM formation of T. koreanum in association with indigenous oak trees. However, distinguishing between the ECM formed by T. koreanum and that formed by other Tuber species remains challenging, underscoring the need for further studies on the formation and characteristics of ECM.


