Before raising to the generic level, Neoerysiphe U. Braun was treated as a section, Galeopsidis, within the genus Erysiphe s. lat. on the basis of the morphology of the ascomatal appendages [1]. The sexual stage of the genus Neoerysiphe is characterized by immature asci in the current season, in which ascospores are formed after the overwintering of chasmothecia. Neoerysiphe galeopsidis (DC.) U. Braun is one of the major globally distributed species of this genus and it encompasses a large number of host plant species, mainly from the family Lamiaceae, besides Acanthaceae, Bignoniaceae, and Malvaceae [2].
Shin & La [3] recorded the anamorph of N. galeopsidis as Erysiphe galeopsidis DC. on Lamium album subsp. barbatum (Siebold & Zucc.) Mennema in 1992 for the first time. However, their record was a list of powdery mildews without description. Although seven additional samples have been collected and examined over the last 30 years, the sexual stage of this fungus was lacking in Korea. Currently, N. galeopsidis has been known as a causal agent of powdery mildew disease on L. album subsp. barbatum, L. amplexicaule L., and L. purpureum L. in Korea [4-6]. In November 2022, the chasmothecia of powdery mildew on L. album subsp. barbatum were collected for the first time in Korea. Here, we describe the morphological characteristics of the holomorph and indicate the phylogenetic position of the fungus based on newly obtained sequences from Korean isolates.
During our field forays, nine samples of L. album subsp. barbatum infected with powdery mildew were collected and preserved at the Korea University Herbarium (KUS). They were deposited as KUS-F10881 (July 14, 1991; Pyeongchang), F19570 (June 11, 2003; Pyeongchang), F20398 (July 1, 2004; Jinju), F20406 (July 2, 2004; Pyeongchang), F23763 (October 9, 2008; Pyeongchang), F24588 (September 14, 2009; Pyeongchang), F28144 (September 15, 2014; Pyeongchang), F29776 (May 25, 2017; Seoguipo), and F33525 (November 8, 2022; Jangseong).
The detailed morphological characteristics of the powdery mildew were observed using an Olympus BX50 microscope (Olympus, Tokyo, Japan). Photomicrographs were captured using a Zeiss AX10 microscope equipped with an AxioCam MRc5 camera (Carl Zeiss, Oberkochen, Germany). All microscopic measurements and characterizations were performed using fresh materials. Photomicrographs of the teleomorphic structures were obtained using a 3% NaOH solution. Thirty measurements were performed for each diagnostic structure.
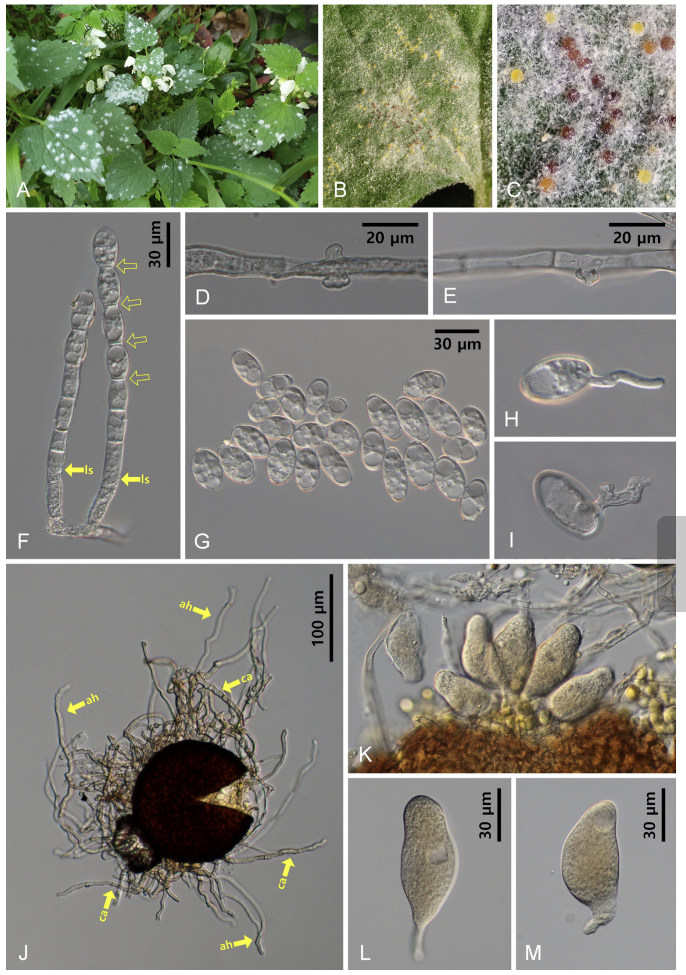
Mycelial mats with conidiophores and conidia were mostly epigenous, less frequently amphigenous, and occasionally cauligenous, forming conspicuous white colonies (Fig. 1A). In the later stages of the disease, chasmothecia formed on both sides of the leaves and occasionally on the stems (Fig. 1B and C). The appressoria on the mycelium were moderately lobed to multi-lobed (Fig. 1D and E). Conidiophores were 86–160(–240) × 10–12 μm, and produced 2–6 immature conidia in chains with a sinuate outline, followed by 1–3 cells (Fig. 1F). The footcells of the conidiophores were straight or slightly swollen, cylindrical, and 26–56 μm long. Conidia were hyaline, cylindrical, oval to ellipsoid, variable in shape and size, 22–42 × 16–22 μm (length/width ratio = 1.2–2.2), occupied by vacuoles, and lacked distinct fibrosin bodies (Fig. 1G). Germ tubes were formed at the perihilar position of the conidia (Fig. 1H and I). Chasmothecia were amphigenous, abundantly epigenous, occasionally cauligenous, scattered to gregarious, dark brown, spherical, and 106–132 μm in diameter, and contained 4–7 asci per chasmothecium (Fig. 1B, C, J and K). Chasmothecial appendages were positioned in the lower half of the chasmothecium; were numerous, with at least 30 in number; mycelioid; usually simple, rarely irregularly branched, interlaced with the aerial hyphae (secondary hyphae) and each other; 0.3–3 times as long as the chasmothecial diameter; 4–7 µm wide; and brown at the base and paler upward (Fig. 1J). Asci were obovoid, saccate, short-stalked, 42–56 × 20–26 µm, and immature in the current year (Fig. 1K–M).
Aerial hyphae were found around the chasmothecia (Fig. 1C and J) and are believed to hold the chasmothecia so as not to be blown away, comparable to the secondary hyphae found in Podosphaera species [2, 4]. Braun & Cook [2] described the chasmothecia of N. galeopsidis as “often forming a dense felt around the ascoma”. Dense felt is regarded as part of the chasmothecial appendages or an overgrowth of the vegetative hyphae [1, 2]. However, in the present study, a “dense felt” was first documented as an aggregate mass of secondary hyphae.
For molecular-phylogenetic analyses, whole cell of genomic DNA was extracted, amplified, and sequenced from KUS-F33525 and F28144. Primer pairs PM3/NLP2 and ITS1/PM6 were used to determine the nucleotide sequences of the large subunit (LSU) of 28S rDNA and the internal transcribed spacer (ITS) region, including the 5.8S rDNA gene, respectively [7]. To obtain consensus sequences, we
Fig. 1
Neoerysiphe galeopsidis, a powdery mildew found on Lamium album subsp. barbatum. (A) Powdery mildew symptoms on the leaves. (B) Chasmothecia of N. galeopsidis formed on the leaf. (C) Close-up view of chasmothecia. Note the white aerial hyphae surrounding the chasmothecia. (D, E) Appresoria formed on the hyphae. (F) Conidiophores. Note the lowest septum (ls) and sinuate outline (open arrows) of the conidiophore. (G) Conidia. (H, I) Conidia in germination. (J) Chasmothecia with appendages (ca) and aerial hyphae (ah). (K) Immature asci protruding from the chasmothecium. (L, M) Immature asci. Note the dense cytoplasm in the asci.
assembled the resulting forward and reverse sequences in MEGA11, and then deposited the combined sequences of ITS + LSU into the DNA Database of Japan (DDBJ) under the following accession numbers: LC761201 for KUS-F33525 and LC761202 for KUS-F28144. We compared our sequences with reference sequences uploaded to GenBank using the BLASTn search tool and found 99.8% identity with sequences of Neoerysiphe galeopsidis (AB498948, AB498946, AB329675, and AB329678) for ITS and LSU. For phylogenetic analysis, a combined ITS + LSU data matrix was created using MEGA11 [8]. Microidium phyllanthi-reticulati Meeboon & S. Takam. was selected as the outgroup, as described by Bradshaw et al. [9]. A phylogenetic tree was generated using PAUP* 4.0, based on the maximum parsimony method with the application of the heuristic search option using the “tree-bisection-reconstruction” (TBR) algorithm. All sites were treated as unordered and unweighted, and gaps were treated as missing data [10]. The strength of the internal branches in the resulting tree was tested using 1,000 replications in bootstrap (BS) analysis. Tree scores, such as tree length, consistency index (CI), retention index (RI), and rescaled consistency index (RC), were calculated. The data matrix consisted of 50 sequences and 1,267 characters, of which 35 (2.76%) were variable and 205 (16.7%) were informative for parsimony analysis. In the consensus tree, the sequences obtained in the present study were placed within the N. galeopsidis group, as supported by an 86% BS value (Fig. 2).
Fig. 2
A maximum parsimony tree of Neoerysiphe spp. derived from a combined data matrix of the ITS + LSU rDNA sequences. The isolates obtained in this study are shown in boldface. Microidium phyllanthi-reticulati was selected as the outgroup. Bootstrap values (> 70%) are indicated on related branches. Calculated tree scores, such as tree length (TL), consistency index (CI), retention index (RI), and rescaled consistency index (RC) were given in the box.

Based on morphological characteristics and molecular-phylogenetic analyses, the powdery mildew on L. album subsp. barbatum was identified as N. galeopsidis. This is the first confirmed report of N. galeopsidis on L. album subsp. barbatum in Korea, providing the holomorph morphology and sequence data for this powdery mildew. Recent studies have revealed that N. galeopsidis represents a species complex, and that some cryptic species that are genetically distinct but morphologically indistinguishable from N. galeopsidis have been described and excluded from this complex. Bradshaw et al. (2022) proposed obtaining new sequences of N. galeopsidis from various host genera of the family Lamiaceae to elucidate this complex. Consequently, the sequences obtained in this study contribute significantly to the expansion of data for further research on this topic.


