INTRODUCTION
Macrofungi play important roles in natural ecosystems, adopting saprophytic, parasitic, and symbiotic lifestyle. They encompass ascomycetes and basidiomycetes, which are characterized by conspicuous sporebearing structures that are easily observable [1]. While the worldwide compilation of macrofungi names by the Index fungorum exceeds 20,000, the recorded macrofungi species in Korea amount to approximately 2,100. Among these, 1,800 species were basidiomycetes fungi and 290 species were ascomycetes fungi [2].
In this study, we conducted taxonomic research on the diversity of wild macrofungi in the forests of South Korea. Four previously unrecorded macrofungal species were collected in 2022. Based on taxonomic identification (morphological features and DNA fungal barcodes), two basidiomycete fungi (Laccaria striatula and Xerula strigosa) and two ascomycete fungi (Leotia atrovirens and Malvipezia emileia) were confirmed. Among these, Malvipezia was discovered to be an unknown genus in South Korea.
Four samples were collected during a mycological survey conducted in 2022 to investigate the mushroom diversity. In this study, these collections were examined for morphological identification based on their macroscopic and microscopic characteristics. The dried materials were mounted in distilled water and 5% KOH using a Zeiss Axio Imager A1 microscope (Jena, Germany) and an Axiocam 503 color camera (Jena, Germany). The taxonomic classification of the studied taxa followed the guidelines provided by the Index Fungorum (http://www.indexfungorum.org). Dried specimens were archived at the herbarium of the National Institute of Biological Resources (NIBR), Incheon, South Korea.
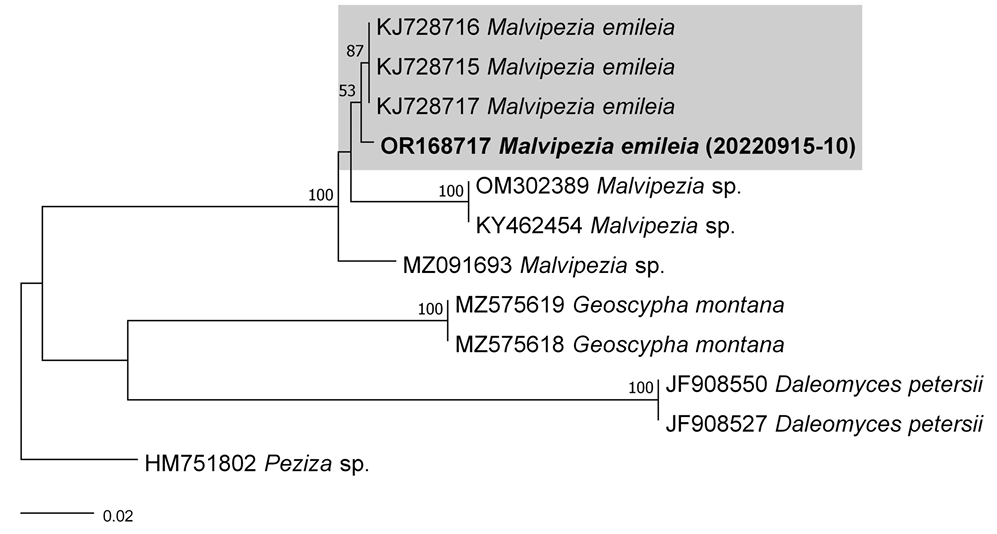
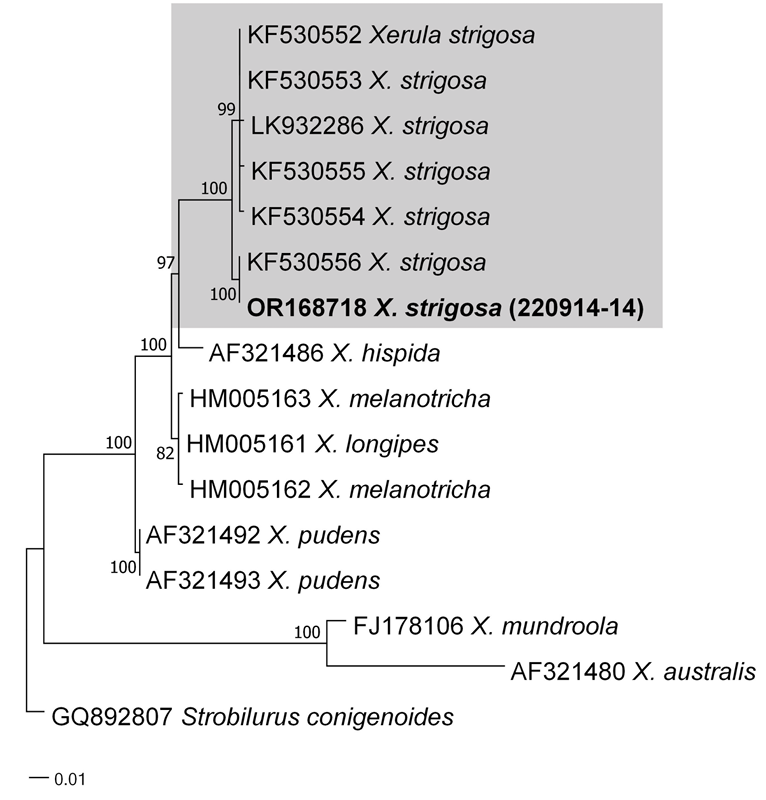
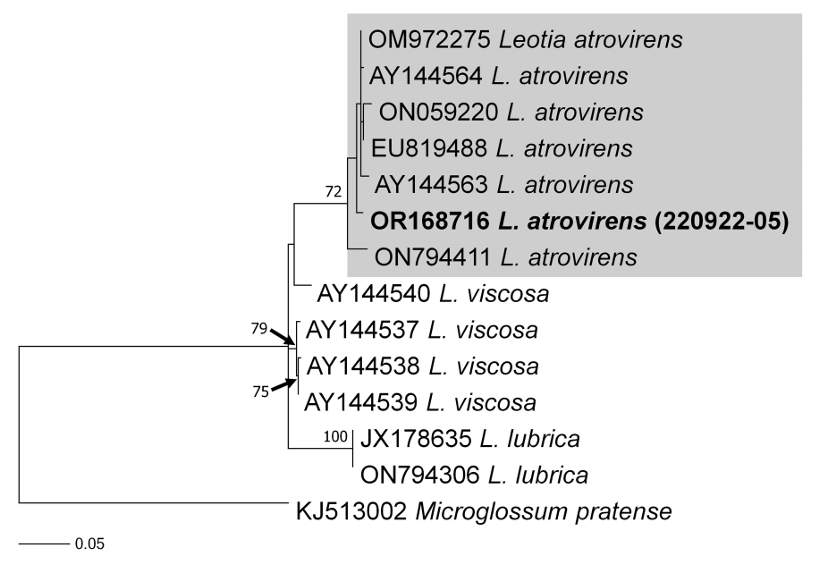
For phylogenetic analysis, genomic DNA was extracted from the specimens using the ZR Genomic DNA Tissue MicroPrep kit (Zymo Research, USA). The ITS region was amplified using a universal primer pair (ITS1 and ITS4). PCR amplicons were purified with the QIAquick Purification Kit (Qiagen, Inc.) and sequenced by Macrogen sequencing service (Macrogen Inc., Seoul, Korea). For phylogenetic study, 61 sequences were download from GenBank (Table 1) [3-21]. The dataset was aligned using MAFFT v.7, with all other parameters set to default values. A Maximum Likelihood (ML) tree of the ITS sequences was constructed using RAxML-HPC2 on XSEDE (v. 8.2.4) [22] through the CIPRES Science Gateway [23]. The robustness of the individual branches was assessed by bootstrapping with 1,000 replicates.As a result, the ITS sequences were subjected to an RaxML analysis, resulting in the resolution of the phylogenetic positions of four species (Laccaria striatula, Leotia atrovirens, Malvipezia emileia, Xerula strigosa) (Figs. 1-4). Each species formed well-supported clades in their respective phylogenetic trees. In Laccaria phylogeny (Fig. 1), the Korean collection grouped together with L. striatula. Within the L. striatula clade, this species exhibited three distinct groups, indicating the presence of a species complex, based on ITS sequences. Further studies are required to validate the phylogenetic position of L. striatula. Regarding the Leotia phylogeny (Fig. 2), the Korean specimen was clustered with L. atrovirens group. Based on the phylogenetic analysis, the Korean collection was conclusively identified as L. atrovirens. In Malvipezia phylogeny (Fig. 3), the Korean collection clustered with M. emileia (KJ728715-KJ728717) with 100% BS values. Thus, the phylogenetic tree we constructed was supported by the position of M. emileia. In Xerula phylogeny (Fig. 4), X. strigosa clade was split into two groups. The Korean specimens clustered with X. strigosa from China (KF530556), with 100% BS values. Further studies are required to confirm this differentiation within X. strigosa clade.
Fig. 1
RAxML tree based on ITS sequences of Laccaria striatula. Sequences generated in this study are indicated in bold text.
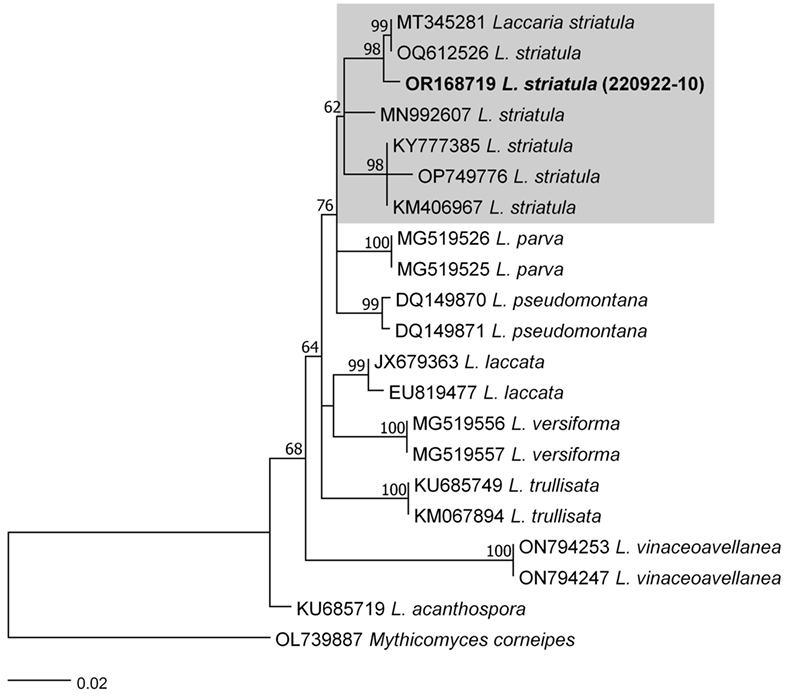
Fig. 2
RAxML tree based on ITS sequences of Leotia atrovirens. Sequences generated in this study are indicated in bold text.
TAXONOMY
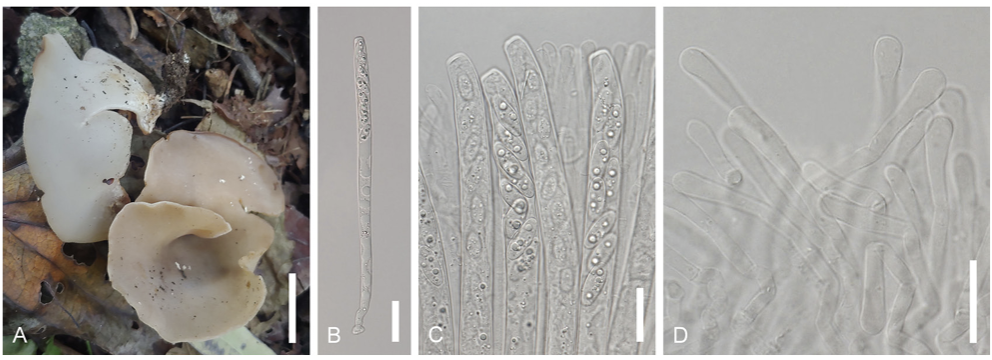
Laccaria striatula (Peck) Peck. Bull. N.Y. St. Mus. 157: 93 (1912) [1911] (Figs. 1 and 5)
Basionym. Clitocybe laccata var. striatula Peck 1897 Korean name: Julmunui-jolgakbeoseot (줄무늬졸각버섯); the epithet ʻstriatula’ originates from the Latin term for little stripes (Latin), referring to pattern of the pileus.Description: Pileus: Diameter ranging from 15 to 40 mm., convex to flattened, with flesh pink to pinkish brown coloration, striated. Lamellae: Adnate, thick, orange-pinkish. Stipe: Colored in shades of orange, pinkish, or brown, finely fibrillose, 20–50 mm × 2–4 mm. Basidia: Clavate-shaped, 4-spored, with dimensions of 53–68 × 12–14.5 μm. Basidiospores: Globose shape, echinulate with spines, measuring 8.012.0 × 9.0–11.5 μm; Q = 0.9–1.3; n = 20. Cheilocystidia rarely observed, filamentous, flexuous. Pileipellis a cutis, hyphae interwoven, cylindrical, bifurcating, clamped. Pileocystidia not observed.
Habitat: Typically found scattered on sand or moss in mixed forest (Pinus koraiensis and Quercus mongolica). Specimen examined: Location: Gangwon-do, Jeongseon-gun, Korea, Coordinates: 37°9ʹ12.3.53ʺN 128°54ʹ24.59ʺE, alt. 1287 m. Collection date: Sep. 22, 2022. Specimen voucher: CKU20220922-10. Remarks. Laccaria striatula is distinguished by its orange-pink to pinkish-brown basidiomata and globose spores with extensive echinulation. The Korean collection have morphologically similar characteristics from specimens of North America (shapes of basidiospores and pileus striations) [24]. This species is the most morphologically similar to L. macrobasidia with size of pileus, basidia, and basidiospore [25]. However, they can be differentiated by the size of stipe (20–50 mm × 2–4 mm vs. 10–50 mm × 0.51.0 mm). In Korea, this species is collected at high elevations (1287 m). Similarly, a collection of L. striatula from the USA (NCBI accession no. KY777385) was collected at a high elevation (1800 m). Therefore, this species may have grown at higher altitudes. Leotia atrovirens Pers. Mycol. eur. (Erlanga) 1:202 (1822) (Figs. 2 and 6) Korean name: jinnoksaek-dugeonbeoseot (진녹색두건버섯); the epithet “atrovirens” derives from the Latin term for dark green, referring to the color of the ascomata. Description: Ascomata: Gelatinous, irregularly rounded or flattened, measuring 6-15 mm in width, with a yellowish to greenish hue, and appearing smooth or furrowed. Stipe: Typically 15-45 mm tall, pale green, and usually roughened. Asci: 8-spored, cylindrical, 120–168 × 7.0–10 μm, tapering towards a long base. Ascospores: Hyaline, ellipsoid, slightly curved, guttulate, measuring 18.0–24.0 × 5.0–6.0 μm; Q = 3.6–4.8; n = 20. Habitat: Found in deciduous forests. Specimen examined: Location: Gangwon-do, Jeongseon-gun, Korea, Coordinates: 37°09ʹ02.49ʺN 128° 54ʹ29.07ʺE, alt: 1316 m. Collection date: September 22, 2022. Specimen voucher: CKU20220922-05. Remarks. According to a previous study [6], L. atrovirens may be L. lubrica parasitized by an asexual fungus, resulting in color change. However, phylogenetic analyses have shown that they are not monophyletic [26]. The constructed phylogenetic tree (Fig. 2) supports the results of this study. Malvipezia emileia (Cooke) Van Vooren, Ascomycete.org 12(4):188 (2020) (Figs. 3 and 7) Basionym: Peziza emileia Cooke 1879 Korean name: Naseon-damjasaekjubalbeoseot (나선담자색주발버섯); derived from the spiralshaped apothecia. The newly designated Korean genus name is Damjasaekjubalbeoseot (담자색주발버섯), derived from the characteristics of light purple apothecia. Description: Apothecia: Cup or spiral shaped, with diameters ranging from 35 to 75 mm. The flesh was waxy, fragile, whitish, weakly brownish, and layered. Asci: Subcylindrical, hyaline, measuring 250–330 × 16.5–18 μm, and containing eight-spores. Ascospores: Ellipsoid-shaped, measuring 16.0–23.0 × 9.0–12.0 μm; Q = 1.7–2.5; n = 20; with warts in the form of irregular ridges. Paraphyses: Subcylindrical, 2.8−4.0 μ m wide at the base, 7–8 μm at the apex, simple, and septate at the tip.Habitat: Found on the ground of the forests.
Specimen examined: Location: Gangwon-do, Wonju-si, Korea. Coordinates: 37°21ʹ47.75ʺN
128°1ʹ19.16ʺE, alt. 452 m. Collection date: Sep. 15, 2022. Specimen voucher: CKU20220915-10. Remarks. There are four species have been recorded in the genus Malvipezia (M. emileia, M. howsei, M. invidula, M. pauli; IndexFungorum.org). Of those, M. emileia is morphologically similar to M. howsei with apothecia color (whitish or weakly brownish vs. brownish-violaceous with slight yellowish), spore size (M. emileia is slightly longer than M. howsei), and ornamentation (warts of M. emileia are slightly larger than M. howsei) [14]. This characteristic of M. emileia is similar to those observed in this study. However, M. howsei contains two distinct clades through this study (Fig. 3). Therefore, more sequences are needed to understand the relationships among this taxon. The genus Peziza sensu lato is further split into four genera (Peziza sensu stricto, Geoscypha, Malvipezia and Phylloscypha), phylogenetically [27]. In Korea, the genus Peziza is also known as Peziza sensu lato. Therefore, a taxonomic study of Peziza sensu lato is needed based on the current research. In the present study, Malvipezia was recorded for the first time in South Korea. Xerula strigosa Zhu L. Yang, L. Wang & G.M. Muell. 2008 (Figs. 4 and 8) Korean name: Ganeunteol-Ppuribeoseot (가는털뿌리버섯); derived from the shape of lean stipe. Description: Pileus: 25–45 mm in diameter, convex to plano-convex, light brown to dark brown. The surface is fibrillose or fine scaly, with an umbonate shape and broad depression over the disc. Lamellae: Cream to white, adnate to adnexed, edges entire. Stipe: 60–110 × 3–4 mm, yellowish-brown, centrally located, pubescent, cylindrical to subcylindrical in shape, tapering towards the base, firm. Basidia: 28.535 × 10.0–12.0 µm, 4–spored, clavate, hyaline. Clamp connections absent in all parts of basidiomata. Basidiospores: Ellipsoidal to broadly ellipsoidal, hyaline, pale yellow, measuring 11.0–15.0 × 10.5–12.5 µm; Q = 1.0–1.4; n = 20. Pleurocystidia: 70–125 × 14–30 µm, with a truncate apex, thick-walled mainly at the base. Pileipellis: hymeniform, 45–62 × 15–20 µm, thick-walled. Habitat: Found on litter and soil in coniferous forest.Specimen examined: Location: Gangwon-do, Wonju-si, Korea. Coordinates: 37°25ʹ12.12ʺN 128°3ʹ13.53ʺE, alt. 322 m. Collection date: Sep. 14, 2022. Specimen voucher: CKU20220914-14.
Remarks. The description based on the Korean collection is in accordance with the holotype description [28]. Xerula strigosa has been documented in both China and Pakistan as well. Hence, considering its morphological features and molecular analysis, this represents the species’ third recorded occurrence.