INTRODUCTION
Recently, dementia diseases have significantly increased because of the increase in elderly over sixty-five years of age in Korea. Some forms of dementia are caused by a lack of neurotransmitters.
Acetylcholine (ACh) is a neurotransmitter in the peripheral nervous system and central nerve system, and a shortage of choline in the brain has been associated with Alzheimer's disease. ACh is converted into choline and acetate by acetylcholinesterase (EC.3.1.1.7, AChE) [1, 2]. Therefore, AChE is a key enzyme in the pathophysiology of dementia, and some drugs that inhibit AChE are used in the treatment of that disease [1] because it reduces the activity of cholinergic neurons. AChE inhibitors (anticholinesterase inhibitors) reduce the rate at which ACh is broken down and hence increase the concentration of ACh in the brain.
Several AChE inhibitors as anti-dementia agents have been extracted and characterized from various plants including Umbilicaria esculenta [3], green tea [4], Securinega suffruticosa [5], Onosma hispida [6], Huperzia serrate [7], Juglans regia [8] and job’s tears [9], etc. However, commercial AChE inhibitors such as Galantamine, Rivastigmine, Donepezil, Tacrine and Memantine have been only approved by the FDA as a drug therapy for dementia [1, 2]. They also have some side effects including nausea and anorexia. Therefore, research on the development of new anti-dementia agents with high efficacy and no side effects is necessary.
The production of useful metabolites of yeast have led to their use in the field of biotechnology [10]. Yeasts are also one of the most widely used model organisms for genetics and cell biology. Yeasts have long been used for the preparation of alcoholic beverages and soy sauces [10], etc. Recently, yeasts have received attention because they have various physiological functionalities such as anti-gout, anti-hypertensive, anti-diabetes, GABA and so on [11-15]. However, little information on bioactive compounds from yeasts is sufficiently available. Therefore, it is necessary to study the development of new bioactive compounds from non-pathogenic, wild yeasts.
For the development of a new anti-dementia agent from non-pathogenic, wild yeasts, screening and characterization of potent anti-dementia AChE inhibitor-producing wild yeasts were investigated and studied for the conditions to produce AChE inhibitors from the selected wild yeasts.
MATERIALS AND METHODS
Microorganisms and chemicals
One hundred seven non-pathogenic, wild yeasts which were isolated from the waters and soils of Daejeoncheon, Yudeungcheon and Gapcheon in Daejeon metropolitan city and midstream of Yeongsangang river in Sangju, Korea in 2017 at the Lab. of Biotechnology, Paichai University, Korea [16] were used for the screening of potent AChE inhibitor-producing yeasts.
The media used in this study, yeast extract-peptone-dextrose (YPD), yeast extract-malt extract (YM) and potato-dextrose (PD) were purchased from the Becton Dickinson (Sparks, MD, USA). Unless otherwise specified, all chemicals and solvents were of analytical grade.
AChE (recombinant human AChE, E.C. 3.1.1.7), acetylthiocholine chloride and 5,5'-Dithiobis (2-nitrobenzoic acid, DTNB) were purchased from the Sigma Chemical Co. (St. Louis, MO, USA). The VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA, USA) was used to measure the AChE activity.
Screening of potent AChE inhibitor-producing yeasts and its microbiological characterization
Yeasts were inoculated in YPD medium (2.0% peptone, 1.0% yeast extract and 2.0% dextrose) and cultured at 30℃ for 24 hr. After centrifugation at 15,000×g for 15 min, the cells were obtained and suspended and washed in distilled water. The cells were resuspended in distilled water and then disrupted by glass bead. After centrifugation at 15,000×g (10 min, 4℃), the AChE inhibitory activities of the supernatant were determined to select the AChE inhibitor - producing yeast.
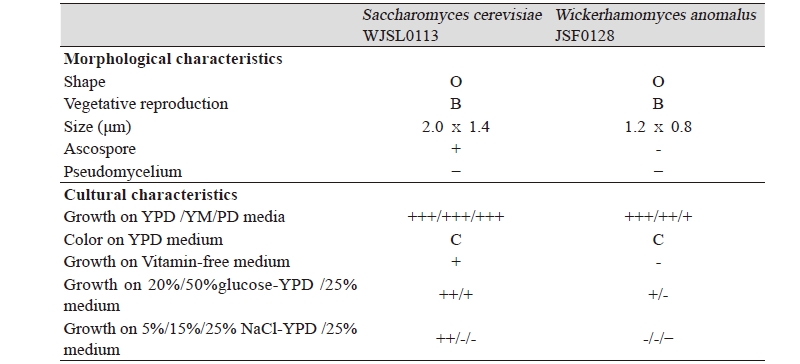
The morphological characteristics of the selected strains including cell shape and size and mode of vegetative reproduction were determined by the microscopy of 24 hr old cultures grown in YPD broth at 28℃ [17]. The other cultural characteristics were investigated according to the methods of the general microbiology lab manual [17].
Assay of the AChE inhibitory activity
The AChE inhibitory activity was measured spectrophotometrically using the technique of Ellman et al. [18]. A mixture of 110 μL of assay buffer (0.1 M sodium phosphate, pH 7.3), 30 μL of AChE (0.8 U/mL), 30 μL of substrate (Acetylthiocholine chloride), 20 μL of 5,5'-Dithiobis (2-nitrobenzoic acid) (DTNB), and 10 μL of sample dissolved in the assay buffer was incubated for 60 min at 37℃. The reaction product 5-thio-2-nitrobenzoate produced enzymatically was measured at 415 nm [2].
The inhibition ratio was obtained by the following equation:
Inhibition (%) = [1-{(S-S0)/(C-C0)}]×100
, where C was the radiation of the control (enzyme, assay buffer, DTNB, and substrate) after 60 min of incubation; C0 was the radiation of the control at time zero; S was the radiation of the tested samples (enzyme, sample solution, DTNB, and substrate) after 60 min of incubation, and S0 was the radiation of the tested samples at time zero.
All data are the mean of duplicated experiments. To check the quenching effect of the samples, the sample solution was added to the reaction mixture C, and any reduction in radiation by the sample was then investigated. The IC50 value was defined as the concentration of the AChE inhibitor that is required to inhibit 50% of the inhibitory (AChE) activity.
Condition for the production of AChE inhibitor
The media and the initial pH, culture temperature and cultivation time for the production of AChE inhibitors from the selected strains were investigated in the range of 15~40℃, initial pH 4.0~9.0 and culture time 0~48 hr by determining the AChE inhibitory activity of each cell free extract.
Assay of anti-dementia using PC12 cell
Construction and cultivation of the recombinant CT105 cell line: PC12 cells (103/mL) that were cultured in the 5% FBS and penicillin/streptomycin-containing RPMI medium were transferred to a 6-well plate and cultured overnight at 37℃. The culture broth consisted of a mixture of A solution (mixture of 2 μg of pCT105 plasmid and 100 μL of serum free medium, SFM) and B solution (mixture of 10 μL of lipofectin transfection reagent and 100 μL of SFM incubated together for 45 min) which was reacted for 15 min After the cells were harvested and washed two times with PBS, they were cultured in 1.5 mL of SFM medium at 37℃ for 6 hr in a CO2 incubator. Then, 5% RPMI medium was added to the cell culture broth and cultured overnight. The cells were subcultured onto fresh 6-well plates containing 450 μg/mL G-418, and a single clone was selected after 2 weeks of culturing.
Induction of neuron apoptosis by recombinant CT105 and repression of apoptosis by cell-free extracts: The repression effect of a cell-free extract from wild yeast on neuron apoptosis was investigated. Three experimental groups were used: WT PC12 cells, recombinant PC12 cells expressing CT105 and recombinant PC12 cells expressing CT105 treated with the cell-free extract. For the cell-free extract treated group, CT105 apoptosis-induced PC12 cells (103/mL) were put into 6-well plates and cultured overnight. Then, 50 μg/mL of the cell-free extracts were added to the wells containing the overnight cultured PC12 cells and RPMI media with 5% FBS and penicillin/streptomycin and then cultured at 37℃ for 24 hr. Finally, all three groups of cells were checked by microscope for apoptosis (×200).
RESULTS AND DISCUSSION
Screening of the AChE inhibitor-producing yeasts
To select potent AChE inhibitor-producing yeasts, cell-free extracts of fifty-three non-pathogenic wild yeasts from the waters and soils of three main rivers in Daejeon metropolitan city including the Daejeoncheon were tested for their AChE inhibitory activities. Only ten wild yeasts were showed AChE inhibitory activity and the other wild yeasts were not determined (Table 1). Among them, Saccharomyces cerevisiae WJSL0113 exhibited the greatest AChE inhibitory activity of 95.2%, and another Saccharomyces cerevisiae WJSL0113 and Saccharomyces cerevisiae WJSL0113 also exhibited a very high inhibitory activity of 84.2% and 53.5%, respectively. However, the other strains exhibited low inhibitory activities below 20% or not determined.
|
Table 1. Acetylcholineseterase inhibitory activities on wild yeasts from three main rivers in Daejeon metropolitan city and Yeongsangang river in Sangju city, Korea 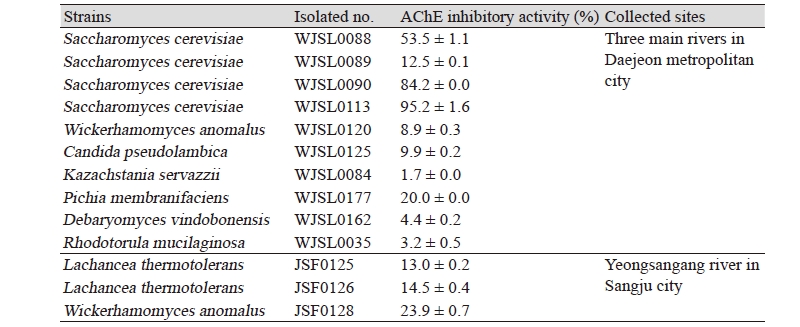
|
|
|
AChE; acetylcholinesterase |
|
Meanwhile, cell-free extracts of eleven non-pathogenic wild yeasts from the waters and soils of Yeongsangang river tested for their AChE inhibitory activities. Only three wild yeasts were showed AChE inhibitory activity and the highest AChE inhibitory activity was observed in Wickerhamomyces anomalus JSF0128 (23.9%) (Table 1). Thermophilic yeasts; Lachancea thermotolerants JSF0125 and JSF0126 exhibited low inhibitory activities of 14.5 and 13.0%, respectively. The AChE inhibitory activities of the cell-free extracts of the wild yeasts Saccharomyces cerevisiae WJSL0113 from the three main rivers in Daejeon metropolitan city were higher than those of the cell-free extracts of the wild yeast Wickerhamomyces anomalus JSF0128 and the thermophilic yeasts Lachancea thermotolerants JSF0125 and JSF0126 from Yeongsangang river in this study.
Furthermore, the AChE inhibitory activity of the cell-free extract from Saccharomyces cerevisiae WJSL0113 also was higher than those of Yarrowia lipolytica S-3 (37.4%) from traditional soybean paste, Doenjang [19], 70% ethanol extract from the edible mushroom Pholiota adiposa fruiting body (30.6%) [20], water extract from walnuts (72.6%) [21], and jobs tears (Coix lachrymajobi L., 55.1%) [9]. Chemical structure and its function of these AChE inhibitors were not yet known with those of our study. Therefore, it is necessary to study further on purification and characterization of AChE inhibitor from the selected yeasts. Finally, we selected Saccharomyces cerevisiae WJSL0113 in the sporogenous wild yeast as a new AChE inhibitor-producing yeast. Wickerhamomyces anomalus JSF0128 was also selected in the asporogenous yeasts because it was not formed ascospore and had sugar-tolerant wild yeast which was grew well in 20% glucose-containing YPD medium. Their microbiological characteristics and conditions for the production of their AChE inhibitors were investigated and compared.
Generally, Saccharomyces cerevisiae has long been used for the preparation of alcoholic beverages [10], bread, etc. It also recently has received much attention because of its various physiological functionalities such as anti-angiogenic [22], anti-hypertensive [23] and anti-dementia [24] agents. There are no studies study on the bioactive compounds from Wickerhamomyces anomalus except for studies on its arabinosidase and xylosidase activities and on improving the aroma development of wine [25]. Therefore, Saccharomyces cerevisiae WJSL0113 and Wickerhamomyces anomalus JSF0128 in this study would be very useful in the anti-dementia medicinal or functional food industry.
Microbiological characteristics of the selected strains,
Microbiological characteristics of the selected strains,
The morphological, cultural characteristics and phylogenetic tree of Saccharomyces cerevisiae WJSL0113 and Wickerhamomyces anomalus JSF0128 are summarized in Table 2 The Saccharomyces cerevisiae WJSL0113 strain formed an oval shaped yeast colony and ascospores. The strain did not form pseudomycelium and grew well in YPD, YM and PD media as well as in vitamin–free YPD medium. The strain was halophilic and thermophilic yeast growing in 10% NaCl-containing YPD medium and at 35℃.
Wickerhamomyces anomalus JSF0128 formed an oval shaped yeast colony and did not form ascospores and pseudomycelium. Like the Saccharomyces cerevisiae WJSL0113, the strain also grew well in YPD, YM and PD media and 10% NaCl-containing YPD. Especially, the yeast strain was sugar–tolerant growing in 20% glucose-containing YPD.
The phylogenetic tree for the selected yeast strains is shown in Fig. 1. Saccharomyces cerevisiae WJSL0113 was closely related to Saccharomyces cerevisiae MG859670.1, KY109393.1 and Saccharomyces ellipsoideus AF005708.1. Wickerhamomyces anomalus JSF0128 was also closely related to Wickerhamomyces anomalus KT895982.1 and KX253664.1. The tree was generated by the neighbor-joining method, using MEGA 7.0.

Fig. 1. Phylogenetic tree of anti-dementia acetylcholinesterase inhibitor-producing yeasts, Saccharomyces cerevisiae WJSL0113 and Wickerhamomyces JSF0128, isolated from freshwater based on the nucleotide sequences of large subunit 28S ribosomal DNA. The tree was generated by the neighbor-joining method, using MEGA7
Conditions for the production of AChE inhibitor
Effect of media; The effects of the medium and its initial pH on the production of AChE inhibitor were investigated. Among the YPD, YM and PD media, cell free extracts from the YPD medium exhibited high AChE inhibitory activities of 92.1% (Saccharomyces cerevisiae WJSL0113) and 40.3% (Wickerhamomyces anomalus JSF0128) although both strains grew well in all media. We investigated the effect of culture temperature and pH. To determine the optimum temperature for the production of AChE inhibitor, Saccharomyces cerevisiae WJSL0113 and Wickerhamomyces anomalus JSF0128 were grown in the YPD medium for 3 days at several temperatures, and then, the AChE inhibitory activity was measured. The optimum temperature for the production of AChE inhibitor in the two strains was 30℃. The initial pH of the medium for the production of AChE inhibitor was also examined in the range of pH 4.0~9.0 (data not shown). In Saccharomyces cerevisiae WJSL0113, the AChE inhibitory activity was very high at 98% in media from pH 6.0 to pH 8.0 and also showed an inhibitory activity of about 60% in the pH 5.0 medium.
However, the highest AChE inhibitory activity of 44% was observed in the pH 5.0 YPD medium for Wickerhamomyces anomalus JSF0128, and this result suggests that Wickerhamomyces anomalus JSF0128 produces more acid–tolerant AChE inhibitor than that of Saccharomyces cerevisiae WJSL0113.
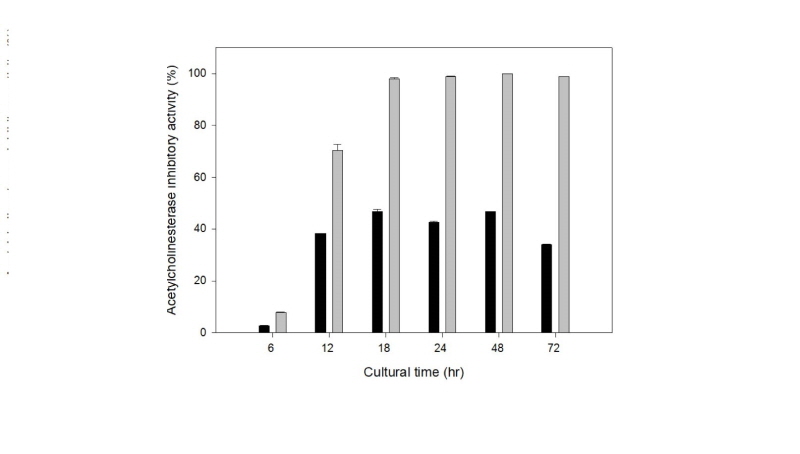
Next, we investigated the effect of the cultivation time. A time course of the production of the AChE inhibitor was determined in a flask culture under the culture conditions for AChE inhibitor production. As shown in Fig. 2, the production of AChE inhibitor in the two strains increased as the cell growth increased, and the maximum production of AChE inhibitor was obtained at 18 hr of cultivation. This cultivation time was shorter than that of Yarrowia lipolytica S-3 from traditional Doenjang (36 hr) [19].
From these results, the culture conditions for the production of the AChE inhibitor from Saccharomyces cerevisiae WJSL0113 is YPD medium (initial pH:6.0), a cultivation time of 18 hr, and a culture temperature of 30℃. The culture conditions for the production of the AChE inhibitor between the two wild yeast strains were not different except for the initial pH of the medium (pH 5.0).
Neuron apoptosis repression (anti-dementia) of the selected wild yeast
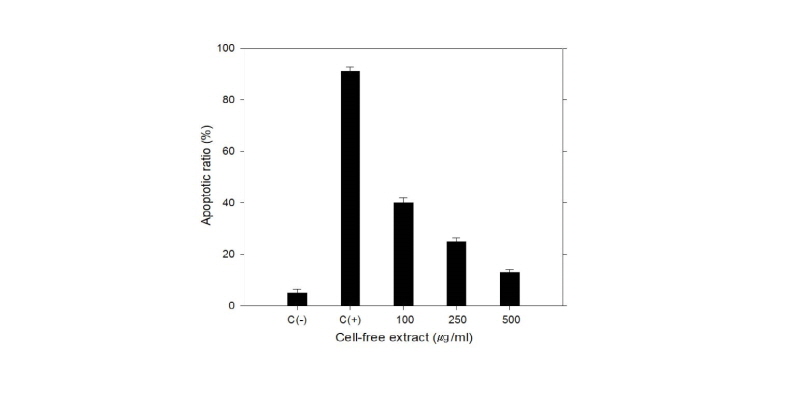
The repression effects of the cell-free extracts from the selected wild yeast on CT105-induced neuron apoptosis were investigated (Fig. 3).
The neuron apoptosis rate was 5.0% (± 1.5%) in the WT PC12 cells and 91.0% (± 1.8%) in the recombinant PC12 cells expressing CT105. However, its apoptosis rate was significantly decreased to 13% (± 1.1%) when treated with the cell-free extract (500 μg/mL). These results suggest that the cell-free extracts from wild yeast in this study repressed or delayed the expression CT105 which is generally induced during dementia.