Bottle gourd (Lagenaria siceraria) is widely used as a rootstock in watermelon cultivation to reduce soil-borne diseases and increase resistance to abiotic stresses [1-3]. Bottle gourds are the most popular rootstocks compared to pumpkin and wild watermelon, because they allow high-quality watermelon production in Korea and Japan [4,5]. In 2020, stem blight symptoms were observed in bottle gourd plants in plastic houses in Wanju, Korea (GPS “35.83840, 127.03734”) (Fig. 1A). The incidence of disease was approximately 30%. Symptomatic plants showed leaf chlorosis, wilting, and progressive death with the infected leaves remaining attached. Sclerotia formed on the stem surface of the diseased plant was observed under a Stemi SV11 stereomicroscope (Carl Zeiss, Oberkochen, Germany) (Fig. 1B).
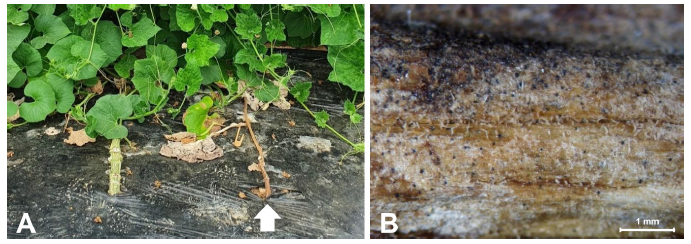
Fig. 1. Symptoms of stem blight (arrow) were observed in bottle gourd plants in the plastic house in Wanju, Korea. (A) Infected plants showing leaf chlorosis and wilting; the plants eventually died with the infected leaves remaining attached. (B) Sclerotia formed on the stem surface of the diseased plant observed under a stereomicroscope
The main fungal pathogens in bottle gourds are Botrytis cinerea (gray mold), Colletotrichum orbiculare (anthracnose), Monosporascus cannonballus (black root rot), Pseudoperonospora cubensis (Downy mildew), Sphaerotheca fusca (Powdery mildew), and Fusarium oxysporum (Fusarium wilt) [6]. Recently, we observed blight symptoms in bottle gourds in plastic houses in Wanju, Korea, thus leading to the identification of the causal agent of blight disease in this study.
Small pieces of stems from lesion margins were sliced into 5 mm sections, surface sterilized with 75% ethanol and 1% sodium hypochlorite for 30 s, and rinsed twice with sterile distilled water. They were placed on water agar plates and the plates were incubated at 25℃ for 3 days. Hyphal tips of the emerging fungus were transferred onto potato dextrose agar (PDA; Difco, Sparks, MD, USA) plates to obtain pure cultures. The mycelia were initially hyaline but gradually became dark with sclerotia after three days. The isolated pathogen was kept in 20% (v/v) glycerol at -70℃ for further study.
Pycnidia and sclerotia were observed under an Axioskop light microscope (Carl Zeiss, Oberkochen, Germany) equipped with an Optinity KCS3-160S digital camera (Korea Lab Tech, Seoul, Korea) and a Stemi SV11 stereomicroscope (Carl Zeiss, Oberkochen, Germany) equipped with an Optinity KCS-31S digital camera (Korea Lab Tech, Seoul, Korea). At least 50 individuals were measured for each structure.
Fungal isolates were grown on PDA at 25℃ for five days. The mycelia of isolated fungi 20G01 were initially hyaline but gradually turned charcoal gray with sclerotia (Fig. 2A). Sclerotia formed abundantly 3 days after inoculation, but spores didn't form until a month of culturing in the PDA medium (Fig. 2B). Black pycnidia and sclerotia were formed on barley leaves on a water agar medium (Fig. 2C). The sclerotia size (n=66) ranged from 52.3 to 123.1 μm in length and 47.7 to 103.1 μm in width (average 80.2×78.1 μm). Pycnidiospores (n=56), 18.7~31.5×9.0~13.5 μm in size, were colorless and without a septum (Fig. 2D). Based on the morphological features, the isolated fungus was assumed to be M. phaseolina [7,8].
Molecular identification and genomic DNA from the isolated fungus 20G01 were extracted. Genomic DNA was extracted using the HiGene Genomic DNA Prep Kit (Biofact, Daejeon, Korea). Four DNA regions were amplified and sequenced, namely the internal transcribed spacer (ITS) region, translation elongation factor 1-α (TEF-1α), β-tubulin (β-TUB), and calmodulin (CAL). The ITS was amplified using ITS5 and ITS4 primers [9], TEF-1α using the EF1-728F and EF1-986R primers [10], β-TUB using the BT2a and BT2b primers [11], and CAL using the CAL-228F and CAL-737R primers [10]. Each amplified fragment was sequenced by a DNA sequencing service (Biofact, Daejeon, Korea) with the same primers used for the amplification and compared with fungal sequences from the National Center for Biotechnology Information using basic alignment search tool (BLAST) algorithms. Along with our sequences, reference sequences of related fungi were used for the phylogenetic analysis. A neighbor-joining tree was constructed using the Tajima-Nei parameter model of MEGA X [12].
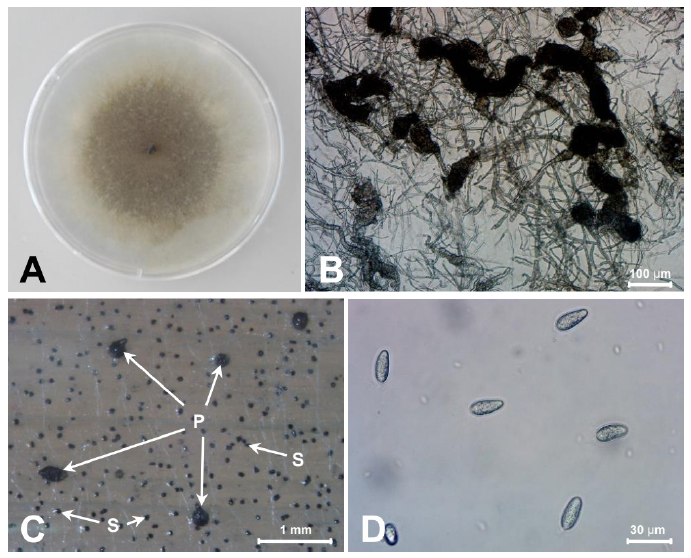
Fig. 2. Morphology of Macrophomina phaseolina isolated from bottle gourd. (A) Fungal colony on a potato dextrose agar (PDA) after 7 days of inoculation at 25℃. The isolates show a black colored colony on the PDA and sclerotia. (B) Sclerotia formed in PDA. (C) Pycnidia (P) and sclerotia (S) formed on barley leaf on water agar medium. (D) Pycnidiospores
The sequences were deposited in GenBank as accession nos. MZ150488 (ITS), MZ358917 (TEF-1α), MZ358915 (β-TUB), and MZ358916 (CAL). A BLAST nucleotide search in the GenBank database indicated that all the four protein sequences had 100% similarity with respective protein sequences of M. phaseolina. Phylogenetic analysis of the ITS region sequence revealed that the 20G01 isolate (accession no. MZ150488) belongs to the M. phaseolina group (Fig. 3). This isolate was deposited in the Korean Agricultural Culture Collection (accession No. KACC 49876).
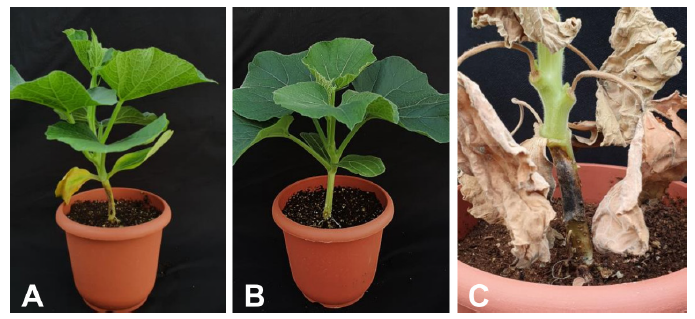
The pathogenicity test was conducted on 4 week-old bottle gourd ‘Bulrojangsaeng’ (Syngenta Korea, Seoul, Korea). The pathogen 20G01 was grown on PDA for 5 days at 25℃ and allowed to colonize a sterile 8 mm wooden toothpick placed on the medium for 10 days. The colonized toothpick was inserted at the bottom of the stem [8]. 15 plants were inoculated with the colonized toothpicks. The control plants were inoculated with sterile toothpicks. The inoculated site was wrapped with water-soaked gauze, and the plants were maintained at 32℃ during the daytime and 25℃ at night in the growth chamber.
Symptoms of stem blight and leaf discoloration were observed 9 days after inoculation, and no symptoms were observed in the control plants (Fig. 4). The fungus was successfully re-isolated from artificially inoculated plants, fulfilling Koch’s postulates.
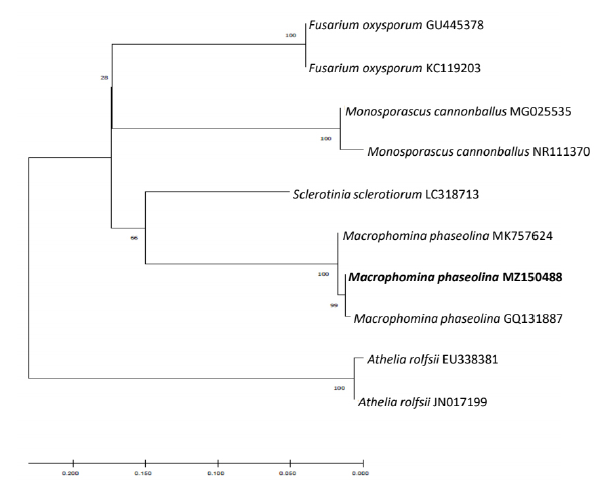
Fig. 3. Phylogenetic analysis of sequence of internal transcribed spacer ribosomal DNA (rDNA) region of isolated fungus and that of other related fungi retrieved from GenBank. The tree was constructed based on the neighbor joining method with 1,000 replicates. The numbers above the branches represent the bootstrap value. The fungus identified in this study is boldfaced
Our results suggest that based on morphology, DNA sequence analysis, and pathogenicity tests, the 20G01 isolate was M. phaseolina.
M. phaseolina infects more than 500 plant species worldwide [6]. It has been reported to cause charcoal rot in Glycine max in Korea [13]. However, this pathogen is not known to infect any species belonging to the genus Lagenaria. To our knowledge, this is the first study reporting charcoal rot in L. siceraria caused by M. phaseolina in Korea.