INTRODUCTION
Endophytic fungi are symbiotic with plants [1]. They live in plant tissues without causing diseases [2]. These fungi chemically defend their host plants against herbivores, insects, and external pathogens [3,4]. Additionally, they enhance the host plants’ resistance to environmental stressors [5]. Endophytic fungi form symbiotic relationships with a range of plants across different climatic zones [6,7]. Their presence is noted in almost all plants, including bryophytes, ferns, conifers, evergreen broad-leaved trees, and deciduous broad-leaved trees [8-12]. These fungi colonize a variety of plant tissues, from vegetative organs, such as roots, stems, and leaves, to reproductive organs, such as flowers and fruits [7]. The relative abundance, species diversity, and community structure of endophytic fungi can vary based on the specific plant tissue [13,14].
Taxus cuspidata Siebold et Zucc. is an evergreen coniferous tree that is geographically distributed in the Far East of Russia, northeastern China, Japan, the Korean Peninsula, and Jeju Island. T. cuspidata grows at altitudes ranging from 700 to 2,500 meters above sea level from Mt. Sungjeoksan in North Korea to Mt. Hallasan on Jeju Island, South Korea [15]. Various tissues of T. cuspidata such as the stem bark, root bark, fibrous roots, twigs, and leaves, produce the anticancer substance taxol (paclitaxel) [16]. Furthermore, endophytic fungi, such as Pestalotiopsis spp. isolated from T. cuspidata, have been shown to produce taxol [17,18].
In this study, we isolated and identified the endophytic fungal strains from T. cuspidata inhabiting Yeongsil area in Mt. Hallasan, Jeju Island. We attempted to confirm the species diversity and community structure according to the plant tissue parts from where the endophytic fungi were isolated.
MATERIAL AND METHODS
Sample collection and fungal isolation
Two to three twig samples with needle leaves of T. cuspidata were collected per tree from the Yeongsil area on Mt. Hallasan in December 2022. We collected samples from 18 individuals and these samples were transported to the laboratory within 24 h. Healthy tissues without disease were selected and surfacesterilized in 30% H2 O2 solution for 2 min and 70% EtOH for 1 min [19]. Three sterilized pieces of the same tissue were placed on potato dextrose agar (PDA; Difco Lab., Detroit, MI, USA) medium. Two media with leaf samples and two media with twig samples were prepared per each individual, and observed while culturing at 25℃. Once the hyphae were confirmed to extend from the tissue, they were sub-cultured in fresh PDA medium for pure culture of the fungal strain.
Morphological characterization
The morphology of the colonies was observed after 7 d of incubation in PDA. The morphology of the unrecorded species was further observed by culturing on malt extract agar (MEA; Kisan Bio, Seoul, Korea) for 7 d. The spores were observed under an optical microscope (Axio Imager A2; Carl Zeiss, Oberkochen, Germany).
Molecular identification
To identify fungal strains, genomic DNA was extracted from the mycelia using the HiGene Genomic DNA Prep Kit (BioFACT, Daejeon, Korea). The internal transcribed spacer (ITS) region containing the 5.8S region of rDNA was amplified using the fungal-specific primers ITS1F and ITS4 [20]. For a more accurate identification of the previously unrecorded fungal species, we amplified the RNA polymerase II second largest subunit (RPB2) region with the specific primers fRPB2-5f and fRPB2-7cR [21]. Polymerase chain reaction products were electrophoresed on a 1.5% agarose gel for 20 min. When an adequate DNA fragment size was confirmed, DNA sequencing was performed (SolGent Co., Ltd., Daejeon, Korea). Fungal species were identified by matching their DNA sequence similarity to that of previously recorded species using BLAST from the National Center for Biological Information. Phylogenetic analysis was conducted to identify unrecorded species. The Neighbor-joining (NJ) method was used for the ITS and RPB2 regions. The Kimura-2-parameter model and 1,000 bootstrap replications were adjusted using the NJ method in MEGA7 [22].
Species diversity
After the species identification, we calculated the relative abundance as follow: To determine species diversity, we calculated Shannon’s diversity index (H´) and species evenness (E) by using MVSP 3.2 software (Kovach Computing Services, Anglesey, Wales, UK). Finally, we compared the relative abundance and the species diversity indices between the needle leaves and the twigs of T. cuspidata.
RESULTS AND DISCUSSION
We isolated a total of 98 endophytic fungal strains from the needle leaves and twigs of T. cuspidata. Molecular identification revealed 49 fungal species across 33 genera (Fig. 1). From needle leaves, 12 species representing 10 genera were isolated, while from the twigs, 43 species from 29 genera were isolated (Table 1).
Fig. 1
Comparison of the isolated endophytic fungal species between the needle leaf and the twig in Taxus cuspidata.
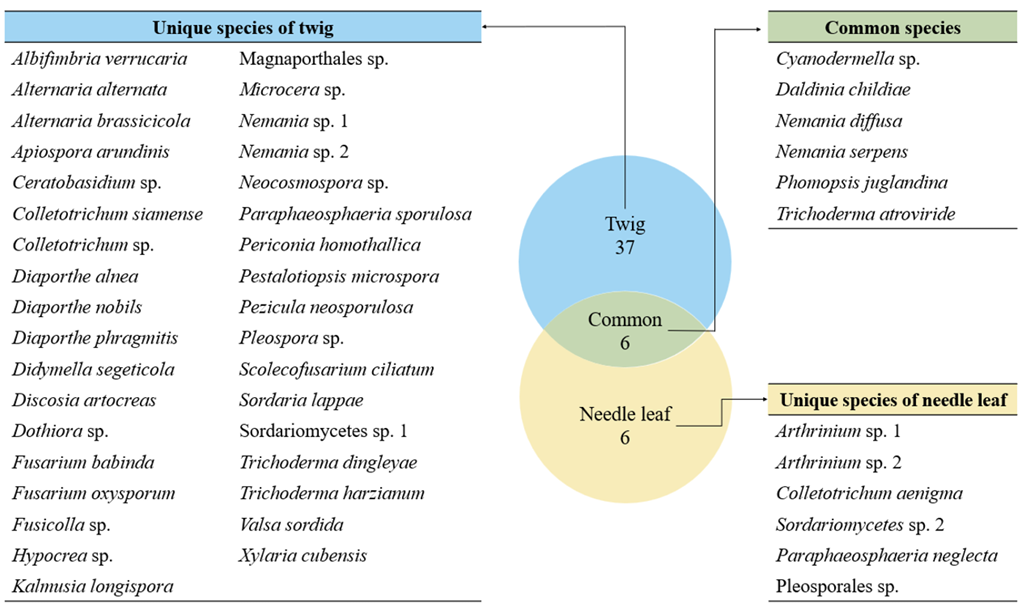
Table 1
The identified endophytic fungal species and the species diversity of endophytic fungal communities.
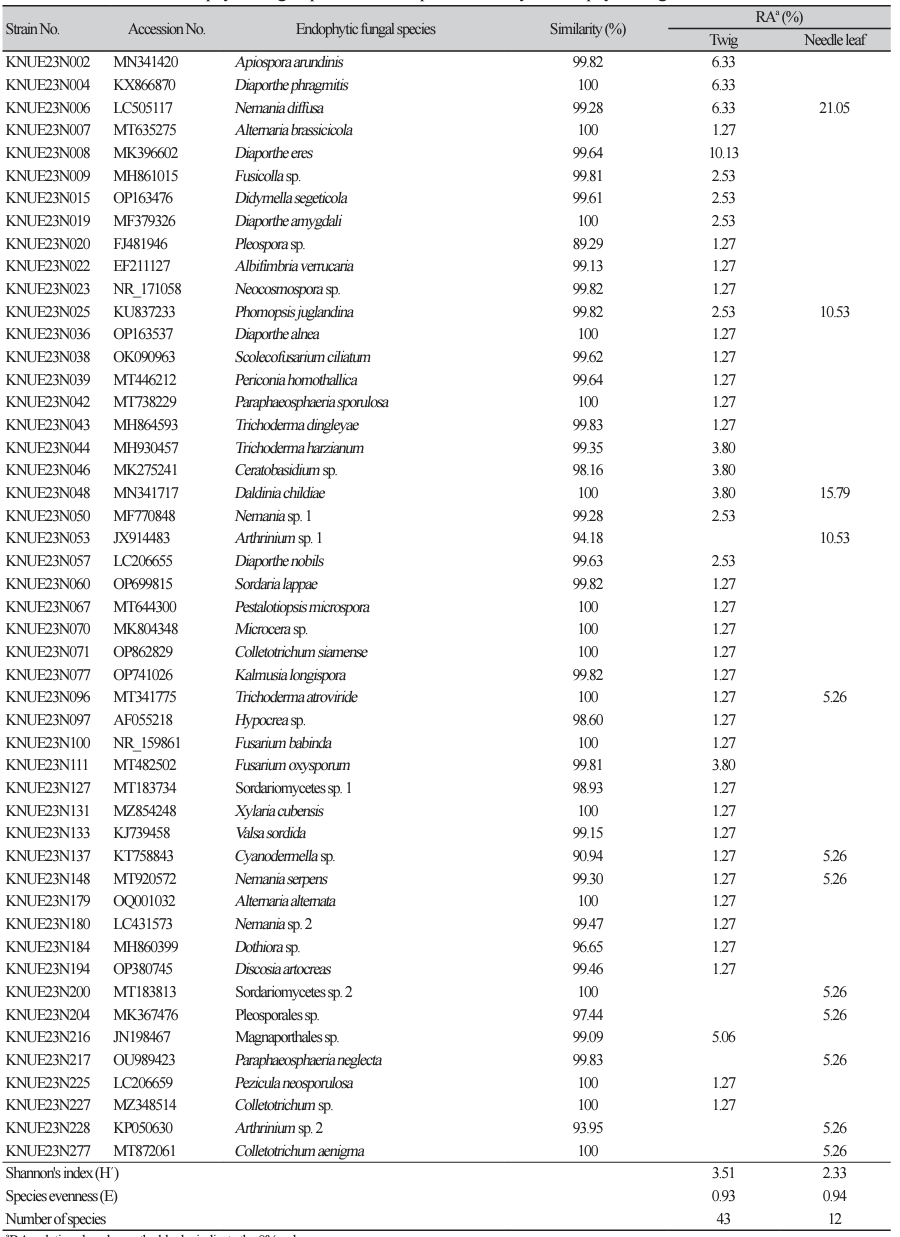
Among the isolated endophytes, only Ceratobasidium sp. belonged to the phylum Basidiomycota, and all other species belonged to the phylum Ascomycota. Six species (Cyanodermella asteris, Daldinia childiae, Nemania diffusa, N. serpens, Phomopsis juglandina, and Trichoderma atroviride) were isolated from both needle leaves and twigs. The remaining species were isolated only from either needle leaves or twigs (Fig. 1). Among the species isolated from needle leaves, N. diffusa showed the highest relative abundance at 21.05%. In twigs, Diaporthe eres showed the highest relative abundance of 10.13%. This suggests that the endophytic fungal communities in needle leaves and twig have distinct species composition, indicating that the plant tissue sites can influence the structure of endophytic fungal community [23].
Of the fungal species isolated, two had not been previously unrecorded in Korea. We describe the morphological characteristics and phylogenetic analysis results for these two fungal strains.
Trichoderma dingleyae Samuels & Dodd, Studies in Mycology 56: 108 (2006) [MB#501036]
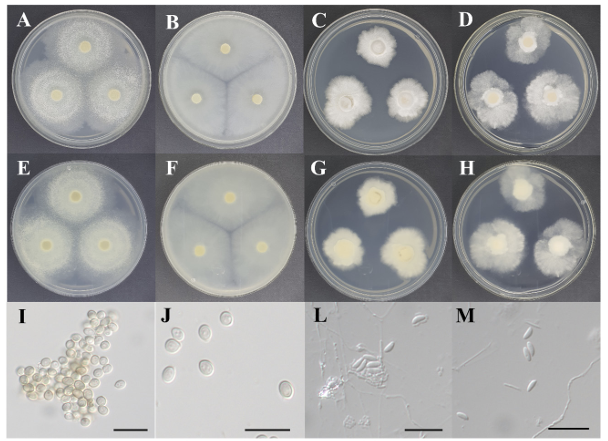
Morphological characteristics: The diameter of the colonies on both PDA and MEA was approximately 45 mm. The colonies on PDA (Figs 2A and E) and MEA (Figs 2B and F) were entirely pale beige or light gray; however, on PDA, the margins showed a paler color pattern because the fluffy aerial mycelium was concentrated in the center. The mycelia exhibited an undulating growth pattern from the center to the margin on PDA, whereas a radial growth pattern was observed on MEA. The colonies showed flat elevations on PDA and MEA. The conidia were hyaline, colorless, or sometimes pigmented and yellowish-brown. They appeared to grow in clusters and exhibited an aseptate, ovoid shape (Figs 2I and J). The conidia were (2.73-) 3.17-3.45 (-3.96)×(2.23-) 2.77-2.98 (-3.31) µm in diameter (n=20).
Fig. 2
Cultural characteristics of two fungal strains. Colonies of Trichoderma dingleyae KNUE23N043 grown for 7 days on potato dextrose agar (PDA) (A: surface, E: reverse) and malt extract agar (MEA) (B: surface, F: reverse), conidia (I, J); Colonies of Xylaria cubensis KNUE23N131 grown for 7 days on PDA (C: surface, G: reverse) and MEA (D: surface, H: reverse), conidia (L, M) (Scale bars=10 μm).
Specimen examined: Yeongsil, Mt. Hallasan, Seogwipo-si, Jeju-do, Republic of Korea; N33°18′54.67″, E126°28′31.058″, December 02, 2022, isolated from the twig of Taxus cuspidata, strain KNUE23N043, NIBR No. NIBRFGC000510444; GenBank accession No. OR689232 (ITS) and OR715106 (RPB2).
Phylogenetic analysis: The DNA sequence from the ITS region of KNUE23N043 showed 99.83% similarity with that of MT530250, whereas the sequence from the RPB2 regene showed 98.07% similarity with that of EU341803. The combined DNA sequence formed a monophyletic group with T. dingleyae strain CBS119056 in the NJ phylogenetic tree (Fig. 3).
Fig. 3
Neighbor-joining phylogenetic tree of Trichoderma dingleyae KNUE23N043 based on the internal transcribed spacer (ITS) and RNA polymerase Ⅱ second largest subunit (RPB2) DNA sequences. Test of phylogeny was 1,000 replicated with a bootstrap method. Hypomyces aurantius denotes an outgroup. The fungal strain isolated in this study is in bold.
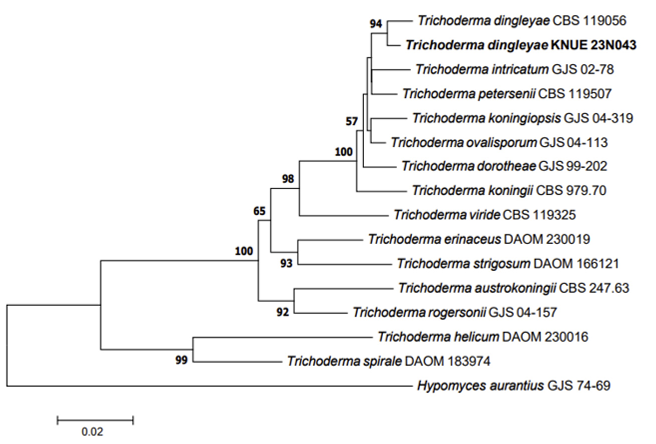
Note: Trichoderma dingleyae was initially isolated from the bark of Nothofagus spp. in New Zealand. This species was an anamorph of Hypocrea dingleyae. This species was derived from various phenotypes of Trichoderma koningii, and was reported as a new species in 2006 [24]. The conidiophores arise laterally from the hyphae and produce broadly ellipsoidal conidia (approximately 4.1-4.3×3.1-3.2 µm) [24]. In the present study, we did not observe any conidiophores; however, the overall morphological characteristics of the conidia we observed were consistent with the original description. The phylogenetic analysis confirmed that T. dingleyae KNUE23N043 can be distinguished from other morphologically related species such as T. koningii, T. dorotheae, and T. intricatum [24]. Based on these analyses, we identified the strain KNUE23N043 as T. dingleyae.
Xylaria cubensis (Mont.) Fr., Nova Acta Regiae Societatis Scientiarum Upsaliensis Ser. 3, 1: 126 (1851) [MB#179243]
Morphological characteristics: The diameter of the colonies on PDA was 29.83±2.30 mm. The colonies were bright white on the surface and beige to ivory on the reverse. The colonies grew radially with irregular margins and increased elevation (Figs 2C and G). The diameter of the colonies on MEA was 37.03 ±1.95 mm. The colonies on both the surface and the reverse were light white. The woolly aerial mycelia were concentrated at the center of the colony, and the substrate mycelia grew radially. The colonies had irregular margins and flat elevations (Figs 2D and H). The conidia were hyaline and colorless and occurred from the lateral side of the hyphae and seemed to form layers because they grew in a sector form (Fig. 2L). They showed an aseptate, ellipsoidal to fusiform shape and were usually curved (Fig. 2M). The conidia were (2.79-) 3.18-3.43 (-3.84)×(1.13-) 1.38-1.51 (-1.81) µm in diam (n=20).
Specimen examined: Yeongsil, Mt. Hallasan, Seogwipo-si, Jeju-do, Republic of Korea; N33°20′19.583″, E126°29′6.673″, 2nd December, 2022, isolated from the twig of Taxus cuspidata, strain KNUE23N131, NIBR No. NIBRFGC000510445; GenBank accession No. OR690434 (ITS) and OR715107 (RPB2).
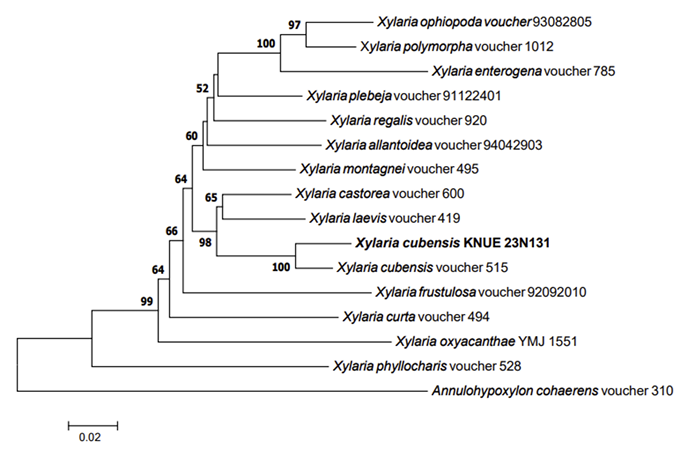
Phylogenetic analysis: The DNA sequence from the ITS region of KNUE23N131 showed 99.08% similarity with that of MF682325, whereas the sequence from the RPB2 regene showed 98.22% similarity with that of MN917802. The combined DNA sequences formed a monophyletic group with X. cubensis voucher 515 in the NJ phylogenetic tree (Fig. 4).
Note: Xylaria cubensis was initially reported as Hypoxylon cubense in 1840, and was later recombined
Fig. 4
Neighbor-joining phylogenetic tree of Xylaria cubensis KNUE23N131 based on the internal transcribed spacer (ITS) and RPB2 DNA sequences. Test of phylogeny was 1,000 replicated with a bootstrap method. Annulohypoxylon cohaerens denotes an outgroup. The fungal strain isolated in this study is in bold.
Pestalotiopsis microspora, Alternaria alternata, and A. brassicola were isolated from the twigs. Both P. microspora and A. alternata have been reported to produce the taxol [18,30]. While Alternaria brassicola can also produce taxol, its known host plant is Terminalia arjuna [31]. Beyond Pestalotiopsis spp. and Alternaria spp., other endophytic fungal species from T. cuspidata also have the potential to produce taxol [17]. Therefore, further screening for taxol production is warranted for the other endophytic fungal species isolated in this study
The Shannon’s index was higher for twigs (H′=3.51) than for needle leaves (H′=2.33). Conversely, species evenness was slightly higher in needle leaves (E=0.93) compared to twigs (E = 0.94), though the difference was not significant. A previous study found higher number of fungal strains in leaves; however, some particular species were dominant and thus, species diversity was found to be higher in the lignified branch bark than in the leaves [13].
In the present study, Ceratobasidium sp., typically found in soil or plant roots, was isolated from a twig of T. cuspidata. This species is commonly recognized as a mycorrhizal or endophytic symbiont in plant roots [32]. While endophytic fungi often undergo horizontal transmission between host plants [33], and some fungal species prefer specific plant tissue parts [34]; however, the presence of Ceratobasidium sp. in twigs suggests potential involvement of vertical transmission in the distribution of endophytic fungi [35,36].
Endophytic fungi and their host plants have a very close evolutionary history, co-evolving through mutual interactions [37]. Studies on the relationship between host plants and endophytic fungal community structures will provide a basis for understanding the interactions between endophytic fungi and their host plants.


